
Statement 1: Cyclopentadienyl anion is much more stable than allyl anion.
Statement 2: Cyclopentadienyl anion is aromatic in nature.
A. Both statement 1 and statement 2 are correct and statement 2 is the correct explanation of the statement-1
B. Both statement 1 and statement 2 are correct but statement 2 is not the correct explanation for statement-1
C. Statement 1 is correct and statement 2 is incorrect.
D. Statement 1 is incorrect and statement 2 is correct.
Answer
598.8k+ views
Hint: To choose the correct option, we should first draw the structure of cyclopentadienyl anion and ally anion. Also check the stability by drawing resonating structures.
Step by step answer:
We should first know about cyclopentadienyl and allyl anion. The cyclopentadienyl anion or cyclopentadienide is an aromatic species with a formula of \[{{\left[ {{C}_{5}}{{H}_{5}} \right]}^{-}}.\]

The above structure is of cyclopentadienyl anion.
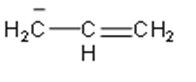
The above structure is of allyl anion.
To check for stability we should first check for resonance in each of the structures. Let us first take the structure cyclopentadienyl anion.
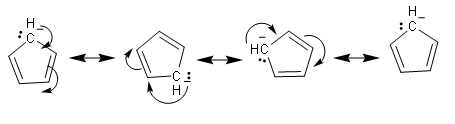
The above structures are the resonating structures of cyclopentadienyl anion. We will also check stability by Huckel rule. This rule estimates whether a planar ring molecule will have aromatic properties. We should note that cyclic ring molecules follow Hückel's rule when the number of its π-electrons equals 4n + 2 where n is a non-negative integer.
So, in the above structure of cyclopentadienyl anion, there are two pi- bonds so there are a total four pi electrons. And there is one negative charge and lone pair. We should only consider negative charge, because lone pair electrons are outside the ring and will not get included in the ring. So, there will be 6 pi electrons. So, cyclopentadienyl anion will follow the Huckel rule and thus it is an aromatic compound.
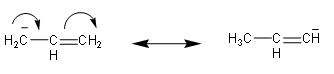
On the other hand in allyl ion, it will also have resonating structure. But it doesn’t show aromatic nature, so, due to this it will be less stable.
Hence, it is now proved that aromatic nature of cyclopentadienyl anion is the reason for its high stability.
Statement 1 and statement 2 are correct, and that’s why option A is the correct answer.
Note: We should know about four criteria for aromaticity. We should check whether the molecule is cyclic. We should check the planarity of the cyclic compounds. The molecule is fully conjugated (p orbitals at every atom in the ring). And the molecule should have 4n+2 π electrons (n=0 or any positive integer).
Step by step answer:
We should first know about cyclopentadienyl and allyl anion. The cyclopentadienyl anion or cyclopentadienide is an aromatic species with a formula of \[{{\left[ {{C}_{5}}{{H}_{5}} \right]}^{-}}.\]

The above structure is of cyclopentadienyl anion.

The above structure is of allyl anion.
To check for stability we should first check for resonance in each of the structures. Let us first take the structure cyclopentadienyl anion.

The above structures are the resonating structures of cyclopentadienyl anion. We will also check stability by Huckel rule. This rule estimates whether a planar ring molecule will have aromatic properties. We should note that cyclic ring molecules follow Hückel's rule when the number of its π-electrons equals 4n + 2 where n is a non-negative integer.
So, in the above structure of cyclopentadienyl anion, there are two pi- bonds so there are a total four pi electrons. And there is one negative charge and lone pair. We should only consider negative charge, because lone pair electrons are outside the ring and will not get included in the ring. So, there will be 6 pi electrons. So, cyclopentadienyl anion will follow the Huckel rule and thus it is an aromatic compound.
On the other hand in allyl ion, it will also have resonating structure. But it doesn’t show aromatic nature, so, due to this it will be less stable.

Hence, it is now proved that aromatic nature of cyclopentadienyl anion is the reason for its high stability.
Statement 1 and statement 2 are correct, and that’s why option A is the correct answer.
Note: We should know about four criteria for aromaticity. We should check whether the molecule is cyclic. We should check the planarity of the cyclic compounds. The molecule is fully conjugated (p orbitals at every atom in the ring). And the molecule should have 4n+2 π electrons (n=0 or any positive integer).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




