
Statement I: Ammonia has a trigonal pyramidal molecular geometry.
Statement II: Ammonia has a tetrahedral electron pair geometry with the three atoms bonded to the central atom.
A. True, true, correct explanation
B. True, true, not correct explanation
C. True, false
D. False, true
Answer
578.4k+ views
Hint: Ammonia is a compound with one nitrogen atom as centre and three hydrogen atoms connected to it. It has three bond pairs of electrons and one lone pair of electrons.
Complete step by step answer:
Before answering this question we need to know everything about ammonia.
Ammonia is a compound of nitrogen and hydrogen with the formula $N{H_3}$ . It is a stable binary hydride and the simplest pnictogen hydride. It is a colourless gas with a characteristic pungent smell. It is a nitrogenous waste particularly among aquatic organisms and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a predecessor to food and fertilizers. It is directly or indirectly a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is both caustic and hazardous in its concentrated form. It is classified as an extremely hazardous substance in the United States and is also subject to strict reporting requirements by facilities which produce, store or use it in significant quantities.
The structure of ammonia is as given below,
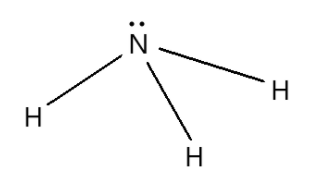
The ammonia molecule has a trigonal pyramidal shape as predicted by the valence shell electron pair repulsion theory with a bond angle of ${106.7^ \circ }$ which is experimentally determined. The central atom nitrogen has five outer electrons with an additional electron from each hydrogen atom. This gives a total of eight electrons or four electron pairs that are arranged in tetrahedral geometry. Three of these electron pairs are used as bond pairs which leaves one lone pair of electrons. The lone pair repel more strongly than bond pairs giving tetrahedral arrangement. Since the lone pairs are invincible the shape of ammonia is trigonal pyramidal.
Now, according to the question both the statements are correct as it has trigonal geometry and a tetrahedral electron pair with three atoms bonded to the central atom.
Therefore, option A is correct.
Note:
Ammonia has four electron pairs and its geometry is based on tetrahedral arrangement of electron pairs. It has three bonded groups and one lone pair. The shape of ammonia is trigonal pyramidal.
Complete step by step answer:
Before answering this question we need to know everything about ammonia.
Ammonia is a compound of nitrogen and hydrogen with the formula $N{H_3}$ . It is a stable binary hydride and the simplest pnictogen hydride. It is a colourless gas with a characteristic pungent smell. It is a nitrogenous waste particularly among aquatic organisms and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a predecessor to food and fertilizers. It is directly or indirectly a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is both caustic and hazardous in its concentrated form. It is classified as an extremely hazardous substance in the United States and is also subject to strict reporting requirements by facilities which produce, store or use it in significant quantities.
The structure of ammonia is as given below,

The ammonia molecule has a trigonal pyramidal shape as predicted by the valence shell electron pair repulsion theory with a bond angle of ${106.7^ \circ }$ which is experimentally determined. The central atom nitrogen has five outer electrons with an additional electron from each hydrogen atom. This gives a total of eight electrons or four electron pairs that are arranged in tetrahedral geometry. Three of these electron pairs are used as bond pairs which leaves one lone pair of electrons. The lone pair repel more strongly than bond pairs giving tetrahedral arrangement. Since the lone pairs are invincible the shape of ammonia is trigonal pyramidal.
Now, according to the question both the statements are correct as it has trigonal geometry and a tetrahedral electron pair with three atoms bonded to the central atom.
Therefore, option A is correct.
Note:
Ammonia has four electron pairs and its geometry is based on tetrahedral arrangement of electron pairs. It has three bonded groups and one lone pair. The shape of ammonia is trigonal pyramidal.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




