
Statement I: Azulene is an example of a non benzenoid aromatic compound.
Statement II: It does not follow Huckel's rule.
(A) Statement I and Statement II are true.
(B) Statement I is true but Statement II is false.
(C) Statement I is false but Statement II is true.
(D) Statement I and Statement II are false.
Answer
512.1k+ views
Hint :We know that the name suggests, non-benzenoid aromatic compounds are those ‘aromatic’ hydrocarbons which do not contain benzene. Any aromatic compound containing multiple rings, with none of them being benzene is a non benzenoid aromatic compound.
Complete Step By Step Answer:
Benzene is an aromatic compound. Almost all benzene containing aromatic compounds are stable in nature. The compounds that do not contain a benzene ring are known as non-benzenoid aromatic compounds, for example Azulene. It is a compound containing two fused rings, seven carbon rings and five carbon rings. It follows all four rules of Aromaticity. As we can see, the compound is cyclic, exhibits conjugation, is planar and has ten pi electrons.
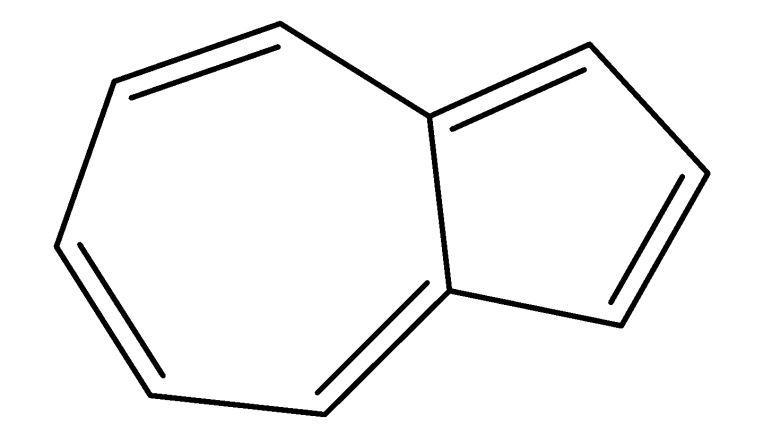
Benzene is a compound which is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each carbon. It is very stable in nature. In organic chemistry, Aromaticity is a unique property of cyclic unsaturated compounds that are very stable thermodynamically. All aromatic compounds follow Huckle’s rule. Here Azulene is a non-benzenoid aromatic compound and it does follow Huckle’s rule.
Therefore, the correct answer is option B.
Note :
Remember the difference between the benzenoid and non benzenoid aromatics as benzenoid in which the name itself suggests that it has at least one benzene ring attached to it that are aromatic in nature whereas non benzenoid are those where there are no benzene rings but are the fused ring system which is aromatic.
Complete Step By Step Answer:
Benzene is an aromatic compound. Almost all benzene containing aromatic compounds are stable in nature. The compounds that do not contain a benzene ring are known as non-benzenoid aromatic compounds, for example Azulene. It is a compound containing two fused rings, seven carbon rings and five carbon rings. It follows all four rules of Aromaticity. As we can see, the compound is cyclic, exhibits conjugation, is planar and has ten pi electrons.

Benzene is a compound which is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each carbon. It is very stable in nature. In organic chemistry, Aromaticity is a unique property of cyclic unsaturated compounds that are very stable thermodynamically. All aromatic compounds follow Huckle’s rule. Here Azulene is a non-benzenoid aromatic compound and it does follow Huckle’s rule.
Therefore, the correct answer is option B.
Note :
Remember the difference between the benzenoid and non benzenoid aromatics as benzenoid in which the name itself suggests that it has at least one benzene ring attached to it that are aromatic in nature whereas non benzenoid are those where there are no benzene rings but are the fused ring system which is aromatic.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




