
How many stereoisomers of $ \text{2 - chloro - 3 - methylbutane} $ exist?
Answer
528.6k+ views
Hint: To know the existence of stereoisomers of $ \text{2 - chloro - 3 - methylbutane} $, we should go through the configuration of $\text{2 - chloro - 3 - methylbutane}$. And then on the basis of configuration, we can conclude the stereoisomers.
Complete step by step solution:
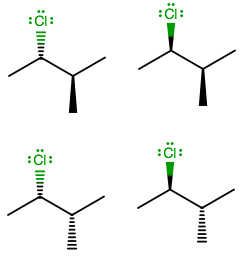
Simply put, there are two configurations per stereo-centre on a compound with all $ s{p^3}\;carbons;\;R\,or\;S $ . So, we rack up stereoisomers according to $ 2 \cdot 2 \cdot 2 \cdot [...] \cdot 2 = {2^n} $ where $ n $ is the number of stereo-centres.
Also, although meso isomers could reduce the number of stereoisomers, there are none here because clearly, $ Cl \ne C{H_3} $ , i.e. there is no plane of symmetry. Therefore, we can ignore meso isomers.
So, there are a total $ {2^2} $ or 4 stereoisomers of $ \text{2 - chloro - 3 - methylbutane} $ exist in nature.
Note:
Stereoisomers are isomers that have the same composition (that is, the same parts) but that differ in the orientation of those parts in space. There are two kinds of stereoisomers: enantiomers and diastereomers. Enantiomers are mirror images, like one’s hands, and diastereomers.
Complete step by step solution:
Simply put, there are two configurations per stereo-centre on a compound with all $ s{p^3}\;carbons;\;R\,or\;S $ . So, we rack up stereoisomers according to $ 2 \cdot 2 \cdot 2 \cdot [...] \cdot 2 = {2^n} $ where $ n $ is the number of stereo-centres.
Also, although meso isomers could reduce the number of stereoisomers, there are none here because clearly, $ Cl \ne C{H_3} $ , i.e. there is no plane of symmetry. Therefore, we can ignore meso isomers.
So, there are a total $ {2^2} $ or 4 stereoisomers of $ \text{2 - chloro - 3 - methylbutane} $ exist in nature.

Note:
Stereoisomers are isomers that have the same composition (that is, the same parts) but that differ in the orientation of those parts in space. There are two kinds of stereoisomers: enantiomers and diastereomers. Enantiomers are mirror images, like one’s hands, and diastereomers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




