
How do the stereoisomers of $ 2-bromo-3-hexene $ arise?
Answer
533.1k+ views
Hint: We know that stereoisomers are the type of isomers which different in the three-dimensional orientations of their atoms in space but have the same molecular formula and the sequence of the bonded atoms. First draw chain structure of the given compound. Then find out all the chiral carbons present in the given compound. Check the number of right and left handed isomers that can be generated for the given structure.
Complete answer:
Stereoisomers are the type of isomers which differ in the three-dimensional orientations of their atoms in space but have the same molecular formula and the sequence of the bonded atoms. It can be of two types- enantiomers and diastereomers. The compound given in the question is $ 2-bromo-3-hexene $
A chiral carbon atom is that carbon atom that is attached to four different types of atoms or groups of atoms. Hence from the structure we can say that only $ C2 $ is the chiral carbon present in the compound. Since there is only one chiral carbon present in the compound hence only two stereoisomers are possible for this structure.
The two stereoisomers can be represented using wedge dash structure. In one wedge dash structure $ Br $ will be present on wedge and $ H $ on dash while in another structure $ Br $ will be present on dash and $ H $ on wedge. The two stereoisomers are:
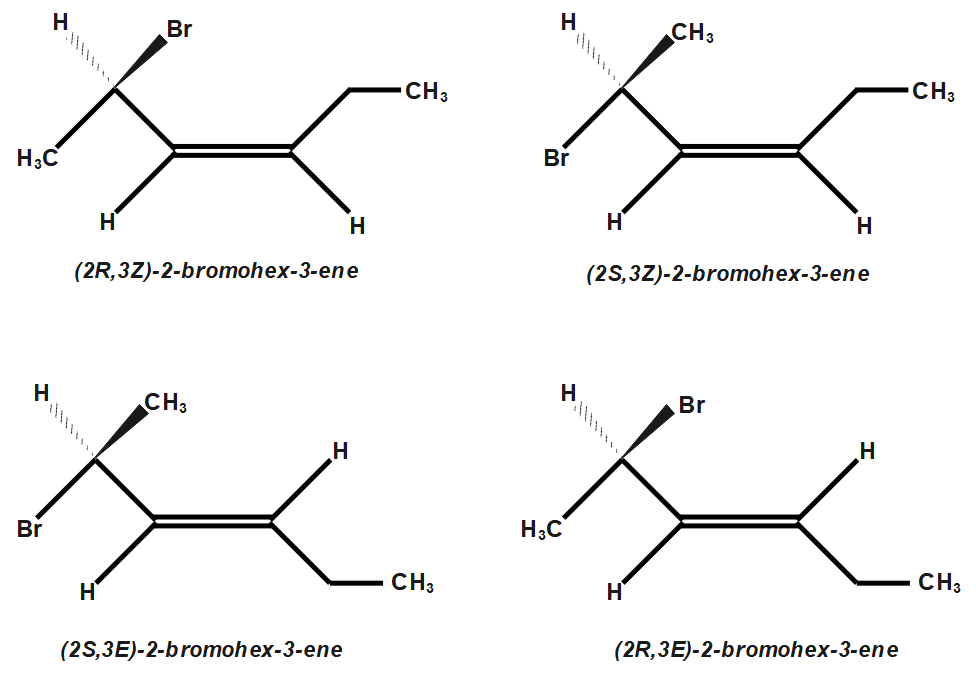
Comparing the two structures we can see that they are mirror images of each other. Since they are non-superimposable mirror images they are known as enantiomers and hence are a type of stereoisomers.
Note:
The total number of stereoisomers of a given compound can also be calculated using $ {{2}^{n}} $ where is the number of chiral centers present in the given compound. The given compound has one chiral center. Using the formula we get that the given compound has $ {{2}^{1}}=2 $ stereoisomers.
Complete answer:
Stereoisomers are the type of isomers which differ in the three-dimensional orientations of their atoms in space but have the same molecular formula and the sequence of the bonded atoms. It can be of two types- enantiomers and diastereomers. The compound given in the question is $ 2-bromo-3-hexene $
A chiral carbon atom is that carbon atom that is attached to four different types of atoms or groups of atoms. Hence from the structure we can say that only $ C2 $ is the chiral carbon present in the compound. Since there is only one chiral carbon present in the compound hence only two stereoisomers are possible for this structure.
The two stereoisomers can be represented using wedge dash structure. In one wedge dash structure $ Br $ will be present on wedge and $ H $ on dash while in another structure $ Br $ will be present on dash and $ H $ on wedge. The two stereoisomers are:

Comparing the two structures we can see that they are mirror images of each other. Since they are non-superimposable mirror images they are known as enantiomers and hence are a type of stereoisomers.
Note:
The total number of stereoisomers of a given compound can also be calculated using $ {{2}^{n}} $ where is the number of chiral centers present in the given compound. The given compound has one chiral center. Using the formula we get that the given compound has $ {{2}^{1}}=2 $ stereoisomers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




