
What is the structural formula of
1. 2-3dibromo-1-phenylpentane
2. 2-oxopropan-1-sulphonate
Answer
575.7k+ views
Hint: Molecular formula tells us about the number of atoms present in the molecule but it does not describe the arrangement of atoms that how atoms are arranged so this concept is defined by the structural formula. It displays how the atoms are organized and attached together in a molecular formula of a chemical compound.
Complete answer:
Structural formula generally describes the structure of any compound and it is further classified into two categories: electron dot structural formula and line bond structural formula. The electron dot structural formula generally uses dots to signify the electrons involved with the bonding between different atoms and the line-bond structural formula makes use of lines and bonds to show the covalent bonds between atoms. Out of these two line bond formulas are frequently used for the representation of arrangement of molecules.
Structural formula of 2-3dibromo-1-phenylpentane can be shown as:
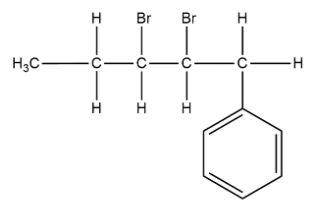
In this compound numbering is starting from right to left on 1st position phenyl group is present and on 2nd and 3rd bromo group is attached and ring is five membered so it is named as 2-3dibromo-1-phenylpentane.
Structural formula of 2-oxopropan-1-sulphonate is shown as:
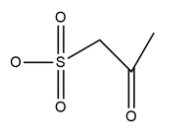
In compound numbering is starting from left to right there is oxo group is present on 2nd position and sulfonate group is on first carbon atom and the chain is 3 carbon atom long so named by propane and the name due to oxo and sulfonate group becomes 2-oxopropan-1-sulphonate.
Note:
Chemical formulas generally have a limited number of symbols due to which they are capable of only limited descriptive power but the structural formulas provide a more complete geometric representation of the molecular structure. Like in the case of isomeric structures chemical formula remains the same but the structure is different so we can say that it is one of most important applications for compounds.
Complete answer:
Structural formula generally describes the structure of any compound and it is further classified into two categories: electron dot structural formula and line bond structural formula. The electron dot structural formula generally uses dots to signify the electrons involved with the bonding between different atoms and the line-bond structural formula makes use of lines and bonds to show the covalent bonds between atoms. Out of these two line bond formulas are frequently used for the representation of arrangement of molecules.
Structural formula of 2-3dibromo-1-phenylpentane can be shown as:

In this compound numbering is starting from right to left on 1st position phenyl group is present and on 2nd and 3rd bromo group is attached and ring is five membered so it is named as 2-3dibromo-1-phenylpentane.
Structural formula of 2-oxopropan-1-sulphonate is shown as:

In compound numbering is starting from left to right there is oxo group is present on 2nd position and sulfonate group is on first carbon atom and the chain is 3 carbon atom long so named by propane and the name due to oxo and sulfonate group becomes 2-oxopropan-1-sulphonate.
Note:
Chemical formulas generally have a limited number of symbols due to which they are capable of only limited descriptive power but the structural formulas provide a more complete geometric representation of the molecular structure. Like in the case of isomeric structures chemical formula remains the same but the structure is different so we can say that it is one of most important applications for compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




