
How many structural isomers are possible for ${{C}_{4}}{{H}_{9}}Br$?
Answer
540.3k+ views
Hint: The structural isomers are also called as the constitutional isomers, which explains that two molecules of concern will have the same molecular formula but will be differently arranged in space which yield different structures.
Complete step-by-step answer:Before going into the solution we should have a brief idea about isomers more specifically about structural isomers.
For the lower classes we are studying about many compounds and its molecular formulae.
Compounds which have the same molecular formulae by different structures are generally called isomers and the phenomenon is called isomerism. It is mainly divided into two categories which are structural isomers and stereoisomers. And again these two divisions are subdivided into many.
So now let us mainly focus on discussing structural isomerism and take the given compound as an example let us draw the various structural isomers associated with the molecule.
Structural isomerism is the phenomena in which the set of molecules of concern will have the same molecular formula but differ in their structural units. Mainly the structures differ by the position of the substituents attached to the parent carbon chain, varies in the position of the double bonds etc.
Now let us draw the structural isomers of ${{C}_{4}}{{H}_{9}}Br$
The compound ${{C}_{4}}{{H}_{9}}Br$ is an alkyl halide and has four carbon atoms so the parent straight chain will be butane and Br is the substituent.
First let's draw the straight chain, the primary alkyl halide.
Since there are four carbon atoms the parent chain will be butane and place the Br in the first carbon of the parent chain.
The straight chain structure is as follows:
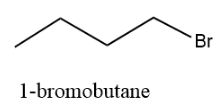
And the IUPAC name of the structure is 1–bromobutane.
Now let us draw the branched structure. First change the position of the substituent and position the Br in the second C of the parent carbon chain.
The structure is as follows:
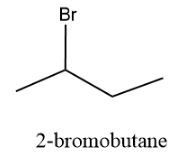
And the compound has the IUPAC nomenclature, 2-bromobutane.
Now let us draw the secondary structure of the alkyl halide, position the methyl group in the second carbon of the parent chain and the corresponding structure is as follows:

Now let us draw the tertiary structure of the alkyl halide and the structure is as follows:
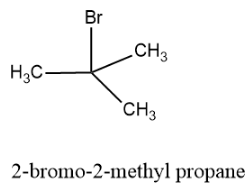
Hence there are four structural isomers possible for ${{C}_{4}}{{H}_{9}}Br$.
Note:We know that for the set of molecules which are isomers will have the same molecular formulae in that sense they will have the same molecular mass. But, the physical properties of the compounds will not be the same for all the molecules, they differ but they are similar in their chemical properties.
Complete step-by-step answer:Before going into the solution we should have a brief idea about isomers more specifically about structural isomers.
For the lower classes we are studying about many compounds and its molecular formulae.
Compounds which have the same molecular formulae by different structures are generally called isomers and the phenomenon is called isomerism. It is mainly divided into two categories which are structural isomers and stereoisomers. And again these two divisions are subdivided into many.
So now let us mainly focus on discussing structural isomerism and take the given compound as an example let us draw the various structural isomers associated with the molecule.
Structural isomerism is the phenomena in which the set of molecules of concern will have the same molecular formula but differ in their structural units. Mainly the structures differ by the position of the substituents attached to the parent carbon chain, varies in the position of the double bonds etc.
Now let us draw the structural isomers of ${{C}_{4}}{{H}_{9}}Br$
The compound ${{C}_{4}}{{H}_{9}}Br$ is an alkyl halide and has four carbon atoms so the parent straight chain will be butane and Br is the substituent.
First let's draw the straight chain, the primary alkyl halide.
Since there are four carbon atoms the parent chain will be butane and place the Br in the first carbon of the parent chain.
The straight chain structure is as follows:

And the IUPAC name of the structure is 1–bromobutane.
Now let us draw the branched structure. First change the position of the substituent and position the Br in the second C of the parent carbon chain.
The structure is as follows:

And the compound has the IUPAC nomenclature, 2-bromobutane.
Now let us draw the secondary structure of the alkyl halide, position the methyl group in the second carbon of the parent chain and the corresponding structure is as follows:

Now let us draw the tertiary structure of the alkyl halide and the structure is as follows:

Hence there are four structural isomers possible for ${{C}_{4}}{{H}_{9}}Br$.
Note:We know that for the set of molecules which are isomers will have the same molecular formulae in that sense they will have the same molecular mass. But, the physical properties of the compounds will not be the same for all the molecules, they differ but they are similar in their chemical properties.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




