
How many structural isomers of molecular formula ${C_3}{H_6}BrCl$ ?
a.) 4
b.) 5
c.) 6
d.) 7
Answer
570.6k+ views
Hint: The structural isomers are the ones which differ in the structure of the molecule. These are the three carbon molecules. By drawing the structure and replacing the Br and Cl with hydrogen, we can get the structural isomers.
Complete answer:
First, let us see what structural isomers are.
The isomers are those species that contain the same molecular formula. The structural isomers are the ones which differ in the structure of the molecule. It is also called constitutional isomers as it is due to different constitutions of the carbon bonds.
The structural isomers are of three different types. The chain isomers in which there is a difference in carbon chains. The other one are the position isomers in which the position of the substituted groups different. The ${C_3}{H_6}BrCl$ have positional isomers. The third one are functional group isomers where there are different functional groups that are formed from the same type of atoms. The ethers, carboxylic acids, aldehydes and ketones form functional isomers.
The ${C_3}{H_6}BrCl$ is a three carbon chain. The various structural isomers that can be drawn are -
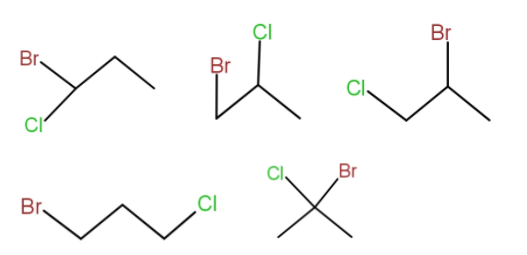
So, the total structural isomers that could be drawn are 5.
Thus, the option b.) is the correct answer.
Note:
It must be noted that structural isomers have the same number of atoms of each type. There is a difference in connectivity of atoms. There is another form known as stereoisomers. These molecules have the same molecular formula but differ in arrangement of atoms in 3D space.
Complete answer:
First, let us see what structural isomers are.
The isomers are those species that contain the same molecular formula. The structural isomers are the ones which differ in the structure of the molecule. It is also called constitutional isomers as it is due to different constitutions of the carbon bonds.
The structural isomers are of three different types. The chain isomers in which there is a difference in carbon chains. The other one are the position isomers in which the position of the substituted groups different. The ${C_3}{H_6}BrCl$ have positional isomers. The third one are functional group isomers where there are different functional groups that are formed from the same type of atoms. The ethers, carboxylic acids, aldehydes and ketones form functional isomers.
The ${C_3}{H_6}BrCl$ is a three carbon chain. The various structural isomers that can be drawn are -

So, the total structural isomers that could be drawn are 5.
Thus, the option b.) is the correct answer.
Note:
It must be noted that structural isomers have the same number of atoms of each type. There is a difference in connectivity of atoms. There is another form known as stereoisomers. These molecules have the same molecular formula but differ in arrangement of atoms in 3D space.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




