
How many structure isomers can you draw for pentane?
(a). 2
(b). 3
(c). 4
(d). 5
Answer
613.5k+ views
Hint: As the name suggests, ‘pent’ means five, thus pentane has five carbon atoms. Structural isomers have the same molecular formula but different bonding patterns. So we can arrange the carbon atoms of pentane to form different structural isomers.
Complete step-by-step solution -
Pentane has three structural isomers and thus option B is the correct one.
• Pentane is an organic compound that falls under the group of alkanes. Organic compounds which do not have any unsaturation (Double or triple bonds) are known as alkanes. The molecular formula of pentane is ${ C }_{ 5 }{ H }_{ 12 }$. The five carbon present in the pentane can be rearranged in three distinct ways to form the three structural isomers of pentane. Let us see the arrangements of the carbon atoms.

• In the second arrangement, four of the carbon atoms in pentane are in the straight-chain and one carbon atom is branched with the second carbon atom of the straight-chain. This isomer is known as iso-pentane and it formula is ${ CH }_{ 3 }{ CH }\left( { CH }_{ 3 } \right) { CH }_{ 2 }{ CH }_{ 3 }$. Let us see the structure of iso-pentane.
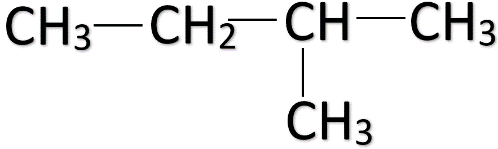
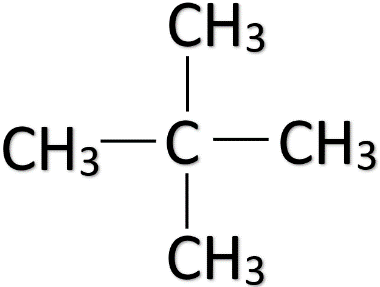
• In the third arrangement, three of the carbon atoms in pentane are in the straight-chain and two carbon atoms are branched with the middle carbon atom of the straight-chain. This isomer is called neo-pentane and its formula is ${ CH }_{ 3 }{ C }{ \left( { CH }_{ 3 } \right) }_{ 2 }{ CH }_{ 3 }$. The structure of neopentane is given below.
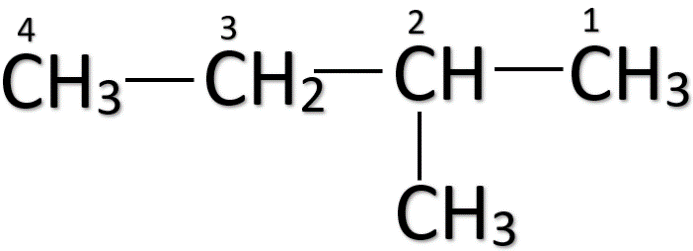
Note: When a carbon atom is branched in a straight-chain, the carbon atoms are numbered from the end carbon which is nearest to the branching carbon. For e.g.
This should be done to make sure that every isomer is distinct. So, don’t confuse between these two structures given below and don’t count them twice while finding out the number of structural isomers.
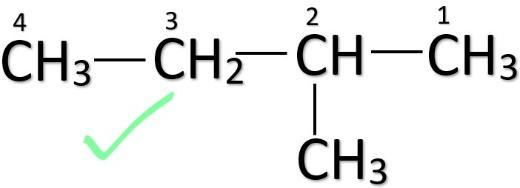
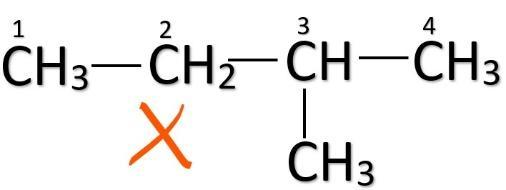
(Correct) (Incorrect)
Complete step-by-step solution -
Pentane has three structural isomers and thus option B is the correct one.
- • Isomers are the molecules that have the same formula but have different and distinct structures. Among all the different types of isomers, structural isomers are those who have different bonding structures but their molecular formula is the same. The bonding structures refers to the way the atoms are bonded in the molecule.
- • In the first arrangement, the carbon atoms are placed in a single line one after another to form a straight-chain. This isomer is called the n-pentane and its formula is ${ CH }_{ 3 }{ CH }_{ 2 }{ CH }_{ 2 }{ CH }_{ 2 }{ CH }_{ 3 }$. The structure of the n-pentane is given below.

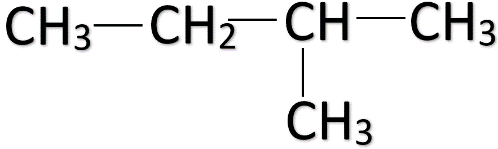

Note: When a carbon atom is branched in a straight-chain, the carbon atoms are numbered from the end carbon which is nearest to the branching carbon. For e.g.

This should be done to make sure that every isomer is distinct. So, don’t confuse between these two structures given below and don’t count them twice while finding out the number of structural isomers.


(Correct) (Incorrect)
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




