
What is the structure of $4 - oxopentanal$ ?
Answer
564.9k+ views
Hint: In the given name, the word ‘pent’ refers to five Carbons so it is clear to s that the given compound is a five-carbon compound. Now using the IUPAC nomenclature, find out the functional groups associated with the suffix and prefix of the given name.
Complete step-by-step answer:The given structure consists of pent i.e. five Carbons. So the basic chain of the compound is
$C - C - C - C - C$
Now in the suffix we have ‘-al’ which associates to a carbonyl group, aldehyde. An aldehyde group is basically oxygen attached to a terminal carbon. So now, we attach an oxygen group to one of the two terminal Carbon atoms.
$C - C - C - C - C = O$
So now the Carbon attached to Oxygen becomes the first Carbon in the chain. It is also given that there is an ‘oxo’ group attached to the fourth carbon. The prefix ‘oxo’ associates to a ketone group which is- Oxygen attached to a secondary Carbon.
It is given to us that the ‘oxo’ group is attached to the fourth carbon. We already know that the Carbon in the aldehyde group is first carbon, so now we attach the ‘oxo’ group to the fourth Carbon to get;
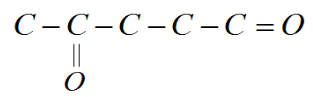
We know that Carbon has four valence electrons and forms four bonds, so we can add hydrogens to the rest of the bonds.
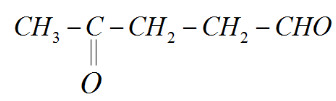
Therefore, this is the structure of $4 - oxopentanal$.
Note: The given compound is a substituted aldehyde. It has five carbons and the aldehyde group in one of the terminals. The term ‘oxo’ is a suffix for the ketone group and it is attached to the fourth Carbon. Therefore we substitute the ketone group on the fourth Carbon.
Complete step-by-step answer:The given structure consists of pent i.e. five Carbons. So the basic chain of the compound is
$C - C - C - C - C$
Now in the suffix we have ‘-al’ which associates to a carbonyl group, aldehyde. An aldehyde group is basically oxygen attached to a terminal carbon. So now, we attach an oxygen group to one of the two terminal Carbon atoms.
$C - C - C - C - C = O$
So now the Carbon attached to Oxygen becomes the first Carbon in the chain. It is also given that there is an ‘oxo’ group attached to the fourth carbon. The prefix ‘oxo’ associates to a ketone group which is- Oxygen attached to a secondary Carbon.
It is given to us that the ‘oxo’ group is attached to the fourth carbon. We already know that the Carbon in the aldehyde group is first carbon, so now we attach the ‘oxo’ group to the fourth Carbon to get;
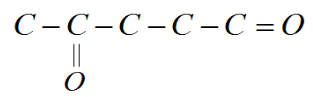
We know that Carbon has four valence electrons and forms four bonds, so we can add hydrogens to the rest of the bonds.
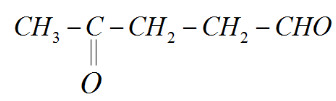
Therefore, this is the structure of $4 - oxopentanal$.
Note: The given compound is a substituted aldehyde. It has five carbons and the aldehyde group in one of the terminals. The term ‘oxo’ is a suffix for the ketone group and it is attached to the fourth Carbon. Therefore we substitute the ketone group on the fourth Carbon.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




