
What structures are formed when water molecules surround individual ions?
Answer
510.6k+ views
Hint :Ions- These are the charged species formed when a neutral atom gains or loses one or more than one electron. There are two types of ions, positively charged ions i.e., cations and negatively charged ions i.e., anions.
Complete Step By Step Answer:
The molecular formula of water is $ {H_2}O $ and its chemical name is dihydrogen monoxide. It is formed when one oxygen atom is covalently bonded to two hydrogen atoms. Due to the presence of lone pair of electrons on the oxygen atom, the water molecule has a bent shape structure as shown below:
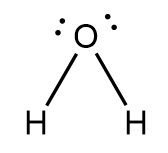
Due to the difference in the electronegativity of oxygen atoms and hydrogen atoms, the water molecule is polar in nature. Due to its polar nature, it has the ability to dissolve various ions and form a structure called hydration sphere around the ions as shown below:
Structure formed by water molecules around negatively charged ions i.e., anions:
As the given ion is negatively charged so, the electropositive part of the water molecule i.e., hydrogen atom will be oriented towards the anions. Due to the polar nature of water molecules, the hydrogen atoms consist of partial positive charge and hence experiences an electrostatic force of attraction with the anions dissolved.
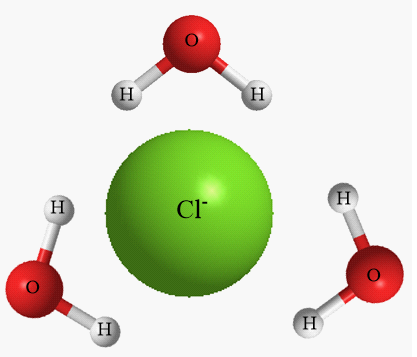
Structure formed by water molecules around positively charged ions i.e., cations:
As the given ion is positively charged so, the electronegative part of the water molecule i.e., oxygen atom will be oriented towards the cations. Due to the polar nature of water molecules, the oxygen atoms consist of partial negative charge and hence experiences an electrostatic force of attraction with the cations dissolved.
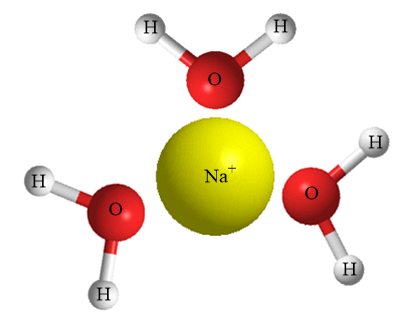
Hence, structures formed when a water molecule surrounds individual ions are known as hydration spheres.
Note :
In water molecules, due to bonding of hydrogen atoms with a highly electronegative element i.e., oxygen atom, the molecule shows intermolecular hydrogen bonding. Due to this, cohesion property is shown by the water molecule.
Complete Step By Step Answer:
The molecular formula of water is $ {H_2}O $ and its chemical name is dihydrogen monoxide. It is formed when one oxygen atom is covalently bonded to two hydrogen atoms. Due to the presence of lone pair of electrons on the oxygen atom, the water molecule has a bent shape structure as shown below:

Due to the difference in the electronegativity of oxygen atoms and hydrogen atoms, the water molecule is polar in nature. Due to its polar nature, it has the ability to dissolve various ions and form a structure called hydration sphere around the ions as shown below:
Structure formed by water molecules around negatively charged ions i.e., anions:
As the given ion is negatively charged so, the electropositive part of the water molecule i.e., hydrogen atom will be oriented towards the anions. Due to the polar nature of water molecules, the hydrogen atoms consist of partial positive charge and hence experiences an electrostatic force of attraction with the anions dissolved.

Structure formed by water molecules around positively charged ions i.e., cations:
As the given ion is positively charged so, the electronegative part of the water molecule i.e., oxygen atom will be oriented towards the cations. Due to the polar nature of water molecules, the oxygen atoms consist of partial negative charge and hence experiences an electrostatic force of attraction with the cations dissolved.

Hence, structures formed when a water molecule surrounds individual ions are known as hydration spheres.
Note :
In water molecules, due to bonding of hydrogen atoms with a highly electronegative element i.e., oxygen atom, the molecule shows intermolecular hydrogen bonding. Due to this, cohesion property is shown by the water molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




