
How many structures are possible for $ {{C}_{5}}{{H}_{8}} $ with one triple bond?
(A) 4
(B) 3
(C) 2
(D) 1
Answer
533.1k+ views
Hint: The atoms can combine or attach to each other by different methods and one of the methods for combining is bonding. The bonding in organic compounds is covalent bonding. There are three different types of bonding and they are single bond, double bond and triple bond. In triple bonds two atoms share three electrons pairs between each other.
Complete step by step answer:
The triple bond is also known as triple covalent bond. ${{C}_{5}}{{H}_{8}} $ is known as pentyne. The structure that can be made of pentyne with one triple bond are:
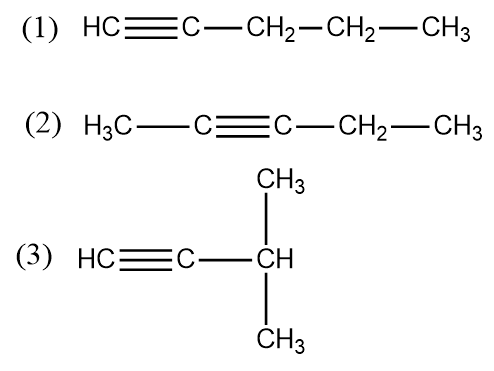
The first structure made is known as Pent-1-yne. The second structure is known as Pent-2-yne.the third structure which is made is branched so we will see the longest chain of carbon atoms and then name it so the name will be 3 methyl but-1-yne. So the structure that can be made by pentyne is 3. So the correct option for this question is option ’b’. in the first structure we have 12 sigma bonds and two pi bonds. In structure two of pent-2-yne we have again 12 sigma bonds and two pi bonds. In structure three of 3 methyl but-1-yne we have again 12 sigma bonds and two pi bonds.
Note:
Sigma and pi bonds are the chemical covalent bonds which are formed by the overlapping of the atomic orbital. in sigma bonds the end to end overlapping takes place. In pi bond the lobes of the atomic orbital are overlapped.
Complete step by step answer:
The triple bond is also known as triple covalent bond. ${{C}_{5}}{{H}_{8}} $ is known as pentyne. The structure that can be made of pentyne with one triple bond are:

The first structure made is known as Pent-1-yne. The second structure is known as Pent-2-yne.the third structure which is made is branched so we will see the longest chain of carbon atoms and then name it so the name will be 3 methyl but-1-yne. So the structure that can be made by pentyne is 3. So the correct option for this question is option ’b’. in the first structure we have 12 sigma bonds and two pi bonds. In structure two of pent-2-yne we have again 12 sigma bonds and two pi bonds. In structure three of 3 methyl but-1-yne we have again 12 sigma bonds and two pi bonds.
Note:
Sigma and pi bonds are the chemical covalent bonds which are formed by the overlapping of the atomic orbital. in sigma bonds the end to end overlapping takes place. In pi bond the lobes of the atomic orbital are overlapped.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




