
Structures of $ N{(C{H_3})_3} $ and $ N{(Si{H_3})_3} $ are different. It is due to the fact that:
(A) Silicon also uses $ d - $ orbitals in multiple bonding.
(B) In case of $ N{(Si{H_3})_3} $ , a lone pair $ N - $ atom is transferred to the empty $ d - $ orbitals of silicon $ (p\pi - d\pi {\text{ overlapping}}) $
(C) Both (A) and (B)
(D) None of the above
Answer
527.1k+ views
Hint: $ Si $ has vacant $ d - $ orbitals while $ C $ has no low-lying $ d - $ orbitals. The lone pair of nitrogen atoms resides on the $ p - $ orbital. There is a $ p\pi - d\pi $ back bonding between nitrogen and silicon atoms.
Complete answer:
Silicon has vacant $ d - $ orbitals and due to this in $ N{(Si{H_3})_3} $ , lone pair of $ N - $ atom is transferred to the empty $ d - $ orbitals of silicon $ (p\pi - d\pi {\text{ overlapping}}) $ with electrons. Hence, $ N{(Si{H_3})_3} $ bond gains partial double bond character and its hybridisation becomes $ s{p_2} $ hybridized. So, it has a trigonal-planar structure. However, there is no such bond in $ N{(C{H_3})_3} $ because $ C $ has no $ d - $ orbital. It undergoes $ s{p_3} $ hybridization and its shape becomes a pyramidal structure.
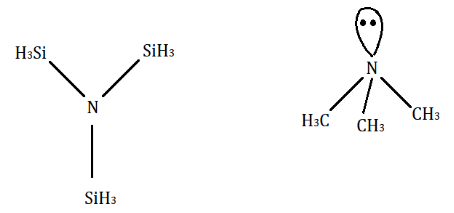
Hence, option C is correct.
Additional Information:
In $ N{(Si{H_3})_3} $ , the electronic configuration of $ Si $ is:
$ 1{s^2},2{s^2}2{p^6},3{s^1}3{p_x}^13{p_y}^13{p_z}^1 $
Hence, $ Si $ has vacant $ d - $ orbitals. The filled $ 2{p^6} $ orbital of nitrogen overlaps with the vacant $ d - $ orbitals of $ Si $ to form $ p\pi - d\pi $ bond. This $ p\pi - d\pi $ bonding is fairly stable.
In Trimethylamine $ (N{(C{H_3})_3}) $ , the lone pair is concentrated on the Nitrogen atom. But in case of Trisilyl amine $ (N{(Si{H_3})_3}) $ , the lone pair on nitrogen gets delocalized onto the three Si atoms bonded to it. Hence, Trimethylamine is a stronger base as compared to Trisilyl amine as it can easily donate its lone pair to other atoms.
Trimethylamine is used in the synthesis of various plant growth regulators, herbicides, dye levelling agents and a number of basic dyes.
Note:
To understand back-bonding just imagine it as a sort of resonance between a lone pair and the other atom with a vacant $ d - $ orbital or $ p - $ orbital. When back bonding is stabilised in $ N{(Si{H_3})_3} $ , its bond angle increases. Thus, the bond length decreases and bond order increases. The bond angle of $ N{(Si{H_3})_3} $ is $ 120^\circ $ while that of $ (N{(C{H_3})_3}) $ is $ 107^\circ $ .
Complete answer:
Silicon has vacant $ d - $ orbitals and due to this in $ N{(Si{H_3})_3} $ , lone pair of $ N - $ atom is transferred to the empty $ d - $ orbitals of silicon $ (p\pi - d\pi {\text{ overlapping}}) $ with electrons. Hence, $ N{(Si{H_3})_3} $ bond gains partial double bond character and its hybridisation becomes $ s{p_2} $ hybridized. So, it has a trigonal-planar structure. However, there is no such bond in $ N{(C{H_3})_3} $ because $ C $ has no $ d - $ orbital. It undergoes $ s{p_3} $ hybridization and its shape becomes a pyramidal structure.

Hence, option C is correct.
Additional Information:
In $ N{(Si{H_3})_3} $ , the electronic configuration of $ Si $ is:
$ 1{s^2},2{s^2}2{p^6},3{s^1}3{p_x}^13{p_y}^13{p_z}^1 $
Hence, $ Si $ has vacant $ d - $ orbitals. The filled $ 2{p^6} $ orbital of nitrogen overlaps with the vacant $ d - $ orbitals of $ Si $ to form $ p\pi - d\pi $ bond. This $ p\pi - d\pi $ bonding is fairly stable.
In Trimethylamine $ (N{(C{H_3})_3}) $ , the lone pair is concentrated on the Nitrogen atom. But in case of Trisilyl amine $ (N{(Si{H_3})_3}) $ , the lone pair on nitrogen gets delocalized onto the three Si atoms bonded to it. Hence, Trimethylamine is a stronger base as compared to Trisilyl amine as it can easily donate its lone pair to other atoms.
Trimethylamine is used in the synthesis of various plant growth regulators, herbicides, dye levelling agents and a number of basic dyes.
Note:
To understand back-bonding just imagine it as a sort of resonance between a lone pair and the other atom with a vacant $ d - $ orbital or $ p - $ orbital. When back bonding is stabilised in $ N{(Si{H_3})_3} $ , its bond angle increases. Thus, the bond length decreases and bond order increases. The bond angle of $ N{(Si{H_3})_3} $ is $ 120^\circ $ while that of $ (N{(C{H_3})_3}) $ is $ 107^\circ $ .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




