
What is surface energy? Establish the relation between the surface tension and surface. energy.
Answer
582.9k+ views
Hint: When we observe a water droplet in a surface it does not look completely spherical. The shape of the droplet is oval. Depending on the surface on which the water droplet is placed the shape changes. This is due to the surface energy. Find out the mathematical expression of both surface energy and surface tension. Compare them to find the relation between them.
Complete answer:
Surface energy can be defined as the energy of the surface of a material. In the bulk of a material the atoms of the material are completely bonded with other atoms and are stable. But at the surface of the material, the atoms have unbalanced bonding i.e. unbalanced set of interactions. Surface energy is the measure of this incomplete bonding of atoms at the surface.
Surface tension can be defined as the attraction of the surface molecules by the atoms in the bulk of the liquid. Surface tension tends to minimize the surface area.
Surface energy can be mathematically expressed as the work done per unit area by the force. So, we can write that the surface energy is work done per unit area or the energy per unit area.
Consider a frame of wire with one side of the rectangle being open or free. Let there be a free arm on the open side of the rectangle and the length of the free arm is l.
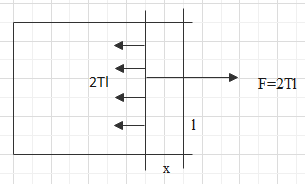
Let there be a soap film on the rectangle. The force on the arm towards the surface or the soap film will be,
$F=T\times 2l$
Where, T is the surface tension of the film.
Let, the force on the free arm moves the free arm inside by a distance of x.
So, the work done in opposing this movement will be,
$W=Fx=2Tlx$
Increase in potential energy of the surface or the soap film can be expressed in terms of the surface energy as,
$EA=E\times 2lx$
Where, E is the surface energy per unit area of the soap film.
This increase in potential energy must be equal to the work done. O, we can write,
$\begin{align}
& 2Tlx=2Elx \\
& T=E \\
\end{align}$
From the above equation we can say that the surface energy of a liquid is equal to the surface tension of the liquid.
Note:
The SI unit of surface tension is $\text{joule}\text{.}{{\text{m}}^{\text{-2}}}$. The surface tension always tends to minimize its surface area. If the surface area of a liquid is needed to be increased, work has to be done. This work done is stored as the potential energy of the surface and is called the surface energy.
Complete answer:
Surface energy can be defined as the energy of the surface of a material. In the bulk of a material the atoms of the material are completely bonded with other atoms and are stable. But at the surface of the material, the atoms have unbalanced bonding i.e. unbalanced set of interactions. Surface energy is the measure of this incomplete bonding of atoms at the surface.
Surface tension can be defined as the attraction of the surface molecules by the atoms in the bulk of the liquid. Surface tension tends to minimize the surface area.
Surface energy can be mathematically expressed as the work done per unit area by the force. So, we can write that the surface energy is work done per unit area or the energy per unit area.
Consider a frame of wire with one side of the rectangle being open or free. Let there be a free arm on the open side of the rectangle and the length of the free arm is l.

Let there be a soap film on the rectangle. The force on the arm towards the surface or the soap film will be,
$F=T\times 2l$
Where, T is the surface tension of the film.
Let, the force on the free arm moves the free arm inside by a distance of x.
So, the work done in opposing this movement will be,
$W=Fx=2Tlx$
Increase in potential energy of the surface or the soap film can be expressed in terms of the surface energy as,
$EA=E\times 2lx$
Where, E is the surface energy per unit area of the soap film.
This increase in potential energy must be equal to the work done. O, we can write,
$\begin{align}
& 2Tlx=2Elx \\
& T=E \\
\end{align}$
From the above equation we can say that the surface energy of a liquid is equal to the surface tension of the liquid.
Note:
The SI unit of surface tension is $\text{joule}\text{.}{{\text{m}}^{\text{-2}}}$. The surface tension always tends to minimize its surface area. If the surface area of a liquid is needed to be increased, work has to be done. This work done is stored as the potential energy of the surface and is called the surface energy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




