
What is the symbol for an atom containing 20 protons and 22 neutrons?
Answer
527.4k+ views
Hint: An atom consists of sub – atomic species such as electrons, protons, and neutrons. Electrons are the negatively charged species, while protons and neutrons are found in the nucleus and protons are positively charged. The number of protons in an atom is equal to the number of electrons which is equal to the atomic number of that atom.
Complete answer:
Symbols or abbreviations are used to denote the elements in the periodic table. These symbols are either derived from the first letter of the element or first two letters of the element. Symbols make the identification and representation of atoms in any equation easier.
An atom contains sub – atomic species. The sub – atomic species are, electrons, protons and neutrons. The number of protons in an atom is equal to the number of electrons, which is equal to the atomic number of any atom. While the sum of protons and neutrons of the atom contribute to the atomic mass of that element.
As the elements in the periodic table are arranged in increasing order of atomic number, having a glance at the periodic table can tell us the atom that contains 20 protons as the number of protons is equal to the atomic number. So looking at the periodic table,
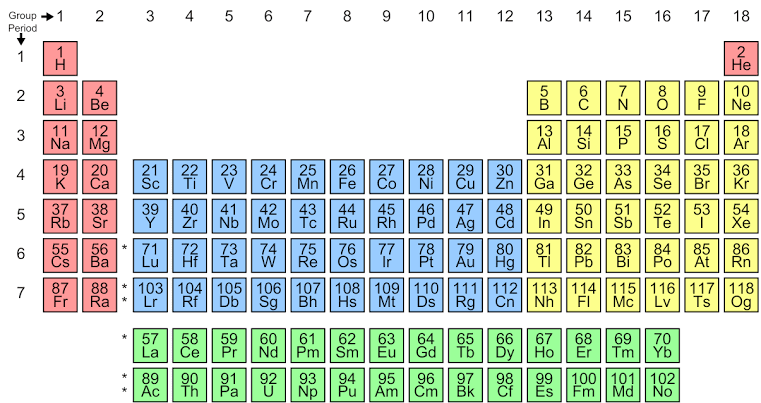
The atom with atomic number 20 is calcium. Hence, the symbol for the atom having 20 protons and 22 neutrons is Ca.
Note:
The calcium atom has atomic number 20 that is the number of protons, while it has a mass number of 42, that represents the number of neutrons as, mass = protons + neutrons. So, neutrons = mass – protons, therefore, 42 – 20 = 22, gives us the number of neutrons which are 22.
Complete answer:
Symbols or abbreviations are used to denote the elements in the periodic table. These symbols are either derived from the first letter of the element or first two letters of the element. Symbols make the identification and representation of atoms in any equation easier.
An atom contains sub – atomic species. The sub – atomic species are, electrons, protons and neutrons. The number of protons in an atom is equal to the number of electrons, which is equal to the atomic number of any atom. While the sum of protons and neutrons of the atom contribute to the atomic mass of that element.
As the elements in the periodic table are arranged in increasing order of atomic number, having a glance at the periodic table can tell us the atom that contains 20 protons as the number of protons is equal to the atomic number. So looking at the periodic table,

The atom with atomic number 20 is calcium. Hence, the symbol for the atom having 20 protons and 22 neutrons is Ca.
Note:
The calcium atom has atomic number 20 that is the number of protons, while it has a mass number of 42, that represents the number of neutrons as, mass = protons + neutrons. So, neutrons = mass – protons, therefore, 42 – 20 = 22, gives us the number of neutrons which are 22.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




