
How would you synthesize 4-methoxyphenol from bromobenzene in not more than five steps? State clearly the reagents used in each step and show the structure of the intermediate compounds in your synthetic scheme.
Answer
509.4k+ views
Hint: Nucleophilic substitution reactions: A class of organic reactions in which a compound that consists of a nucleophile termed as leaving group interacts with an external nucleophile which replaces the leaving group in the compound and removal of leaving group takes place as a nucleophile.
Complete answer:
The reaction mechanism takes place as follows:
Step-1: As nucleophilic substitution reaction on benzene ring is not an easy task, so bromobenzene reacts with sodium hydroxide under high pressure and high temperature to give phenol. The reaction is given as follows:
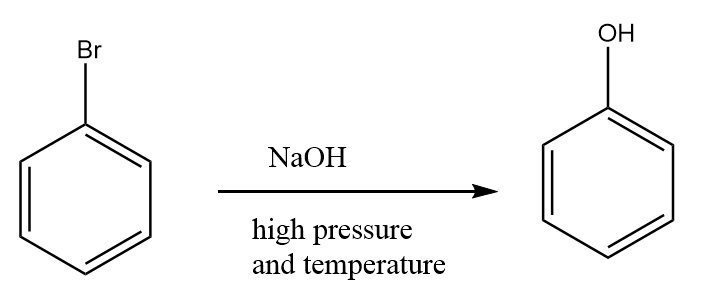
Step-2: Phenol further reacts with $C{H_2}{N_2}$ to form anisole along with the removal of nitrogen gas. The reaction proceeds as follows:
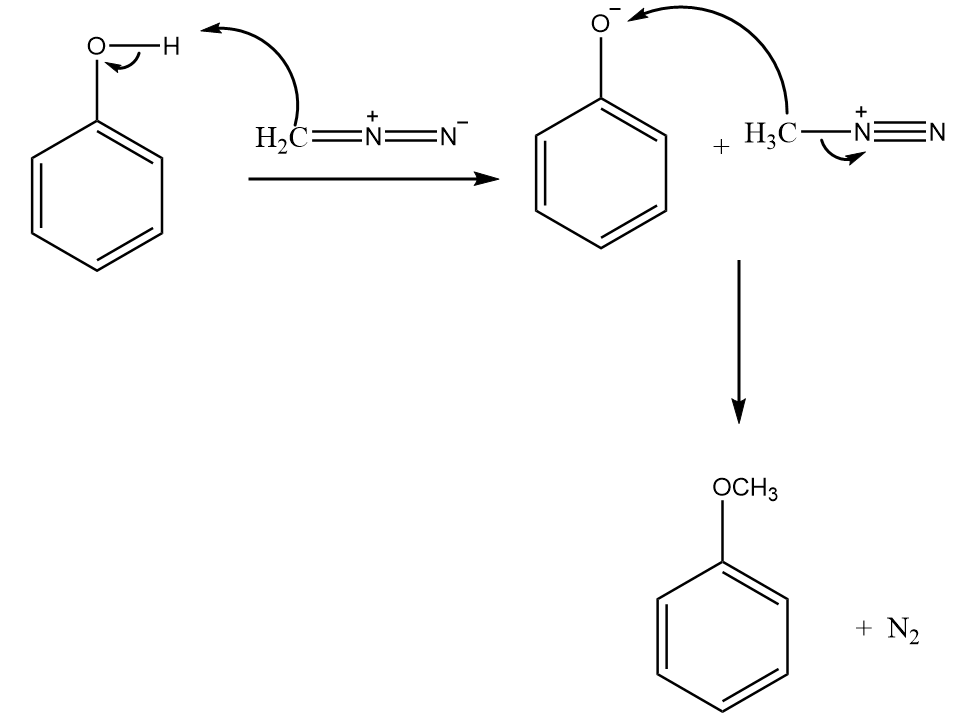
Step-3: Because $OC{H_3}$ is an ortho-para directing group so, anisole on reaction with concentrated sulphuric acid gives para- methoxy benzene sulphonic acid as the major product. The reaction proceeds as follows:
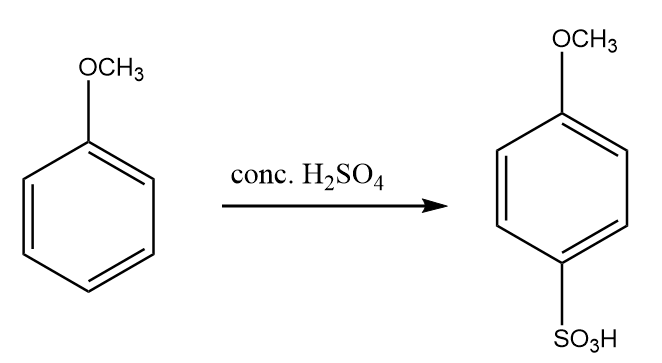
Step-4: para- methoxy benzene sulphonic acid when fused with sodium hydroxide, then the formation of sodium salt of methoxy phenol takes place along with the removal of sodium sulfite and water. The reaction proceeds as follows:
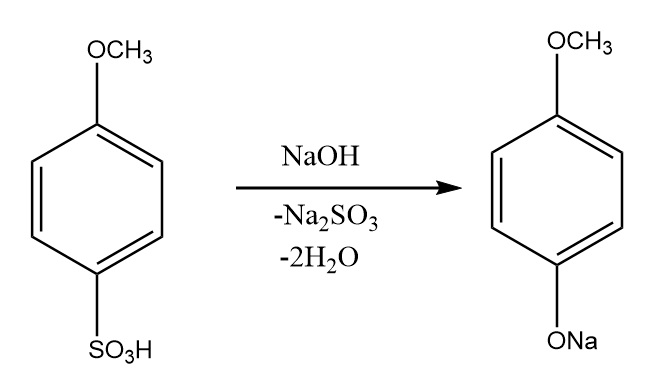
Step-5: On hydrolysis reaction of sodium salt of methoxy phenol in acidic medium, we obtain 4-methoxy phenol as the major product. The reaction proceeds as follows:
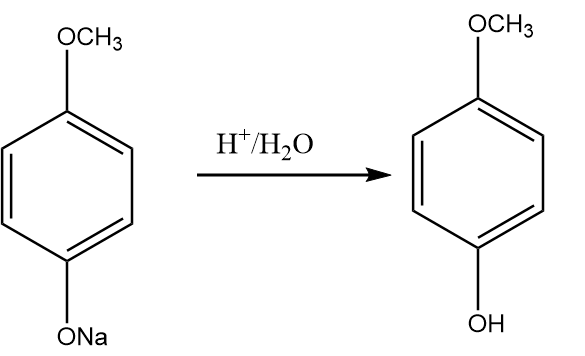
Hence, with the help of the given reaction sequence 4-methoxy phenol is synthesized from bromobenzene within five steps.
Note:
It is important to note that although $OC{H_3}$ is an ortho-para directing group i.e., it has a tendency to form both the compounds but in most of the reactions, the formation of para products takes place in majority. Also, fused sodium hydroxide in the reaction sequence simply refers to its molten form i.e., $NaOH$ is reacted with the compound in its molten state.
Complete answer:
The reaction mechanism takes place as follows:
Step-1: As nucleophilic substitution reaction on benzene ring is not an easy task, so bromobenzene reacts with sodium hydroxide under high pressure and high temperature to give phenol. The reaction is given as follows:

Step-2: Phenol further reacts with $C{H_2}{N_2}$ to form anisole along with the removal of nitrogen gas. The reaction proceeds as follows:

Step-3: Because $OC{H_3}$ is an ortho-para directing group so, anisole on reaction with concentrated sulphuric acid gives para- methoxy benzene sulphonic acid as the major product. The reaction proceeds as follows:

Step-4: para- methoxy benzene sulphonic acid when fused with sodium hydroxide, then the formation of sodium salt of methoxy phenol takes place along with the removal of sodium sulfite and water. The reaction proceeds as follows:

Step-5: On hydrolysis reaction of sodium salt of methoxy phenol in acidic medium, we obtain 4-methoxy phenol as the major product. The reaction proceeds as follows:

Hence, with the help of the given reaction sequence 4-methoxy phenol is synthesized from bromobenzene within five steps.
Note:
It is important to note that although $OC{H_3}$ is an ortho-para directing group i.e., it has a tendency to form both the compounds but in most of the reactions, the formation of para products takes place in majority. Also, fused sodium hydroxide in the reaction sequence simply refers to its molten form i.e., $NaOH$ is reacted with the compound in its molten state.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




