
Tetraethyl lead is used as
A. Fire extinguisher
B. Mosquito repellent
C. Petroleum additive
D. None of the above
Answer
569.4k+ views
Hint: Tetraethyl lead shows the presence of ethyl groups and lead atom. In tetraethyl lead, lead is the central metal atom and ethyl groups are attached to it. Also it is an organo lead compound.
Complete step by step answer:
The structure of tetraethyl lead is given below as follows:
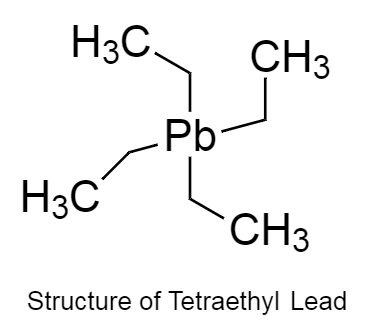
The structure of tetraethyl lead is tetrahedral.
The chemical formula of tetraethyl lead is ${C_8}{H_{20}}Pb$ .
There are four ethyl groups present attached to a single lead atom.
The IUPAC name of tetraethyl lead is tetraethylplumbane.
It has a sweet odor and is a viscous colorless liquid.
It is soluble in water.
It causes lots of pollution in the environment from the smoke released from the vehicles.
The other name of tetraethyl lead is lead tetraethyl.
Tetraethyl lead acts as an anti knocking agent in gasoline and jet fuel and is added to petrol in order to reduce the ignition of vapors of petrol.
Thus, tetraethyl lead is a petroleum additive.
So, the correct answer is Option C.
Note: Tetraethyl lead is prepared by reacting chloromethane and sodium-lead alloy in the presence of a catalyst.
The compounds related to tetraethyl lead are tetraethyl methane, tetraethyl tin and tetraethyl germanium.
tetraethyl lead acts as a negative catalyst. If we inhale tetraethyl lead through the skin it will lead to acute or chronic poisoning. It is banned all over the world except some countries which still use it. The other anti knocking agent used besides tetraethyl lead is methyl cyclopentadienyl manganese tricarbonyl.
Complete step by step answer:
The structure of tetraethyl lead is given below as follows:

The structure of tetraethyl lead is tetrahedral.
The chemical formula of tetraethyl lead is ${C_8}{H_{20}}Pb$ .
There are four ethyl groups present attached to a single lead atom.
The IUPAC name of tetraethyl lead is tetraethylplumbane.
It has a sweet odor and is a viscous colorless liquid.
It is soluble in water.
It causes lots of pollution in the environment from the smoke released from the vehicles.
The other name of tetraethyl lead is lead tetraethyl.
Tetraethyl lead acts as an anti knocking agent in gasoline and jet fuel and is added to petrol in order to reduce the ignition of vapors of petrol.
Thus, tetraethyl lead is a petroleum additive.
So, the correct answer is Option C.
Note: Tetraethyl lead is prepared by reacting chloromethane and sodium-lead alloy in the presence of a catalyst.
The compounds related to tetraethyl lead are tetraethyl methane, tetraethyl tin and tetraethyl germanium.
tetraethyl lead acts as a negative catalyst. If we inhale tetraethyl lead through the skin it will lead to acute or chronic poisoning. It is banned all over the world except some countries which still use it. The other anti knocking agent used besides tetraethyl lead is methyl cyclopentadienyl manganese tricarbonyl.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




