
The acid present in vinegar is-
A. formic acid
B. acetic acid
C. citric acid
D. malic acid
Answer
565.5k+ views
Hint: As we know vinegar is obtained by a process called fermentation. that It is found that for the production of vinegar, ethanol or we can say ethyl alcohol is fermented by bacteria from the family called Acetobacteraceae.
Complete answer:
- We can easily define vinegar as a dilute solution of ethanoic acid in water. It is also called acetic acid. It is found that vinegar is a naturally occurring compound which is in the liquid state, hence there is no defined concentration for it. The chemical formula of vinegar is $C{{H}_{3}}COOH$ .
- It is found that the concentration of acetic acid in water is of the range 5% and 20%.
- As we know that vinegar is mainly a diluted solution of acetic acid in water. Due to this reason vinegar is considered as a weak acid. It is found that acid present in vinegar is an organic aliphatic carboxylic acid with two carbon atoms.
- We can draw the molecular structure of vinegar as:
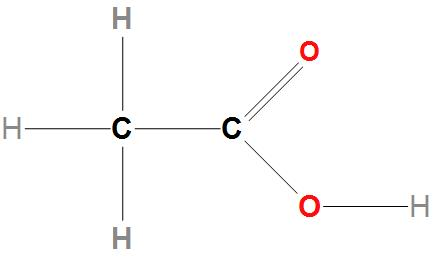
Hence, we can conclude that the correct option is (b), that is the acid present in vinegar is acetic acid .
Note:
- As we know that the acidity of this compound is low, that is it has a very low value of pH, but still the acetic acid does not show complete dissociation in water.
Complete answer:
- We can easily define vinegar as a dilute solution of ethanoic acid in water. It is also called acetic acid. It is found that vinegar is a naturally occurring compound which is in the liquid state, hence there is no defined concentration for it. The chemical formula of vinegar is $C{{H}_{3}}COOH$ .
- It is found that the concentration of acetic acid in water is of the range 5% and 20%.
- As we know that vinegar is mainly a diluted solution of acetic acid in water. Due to this reason vinegar is considered as a weak acid. It is found that acid present in vinegar is an organic aliphatic carboxylic acid with two carbon atoms.
- We can draw the molecular structure of vinegar as:

Hence, we can conclude that the correct option is (b), that is the acid present in vinegar is acetic acid .
Note:
- As we know that the acidity of this compound is low, that is it has a very low value of pH, but still the acetic acid does not show complete dissociation in water.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




