
The acid which contains a peroxo linkage is:
A.Sulphurous acid
B.Pyrosulphuric acid
C.Dithionic acid
D.Caro’s acid
Answer
582.3k+ views
Hint: Peroxo linkage is an oxygen-oxygen bond present in any compound. To know this answer we need to see if peroxy bond is present in the compounds given in the options or not. This can be known from the structure of the acids.
Step by step answer:
First, we need to know about perexo linkage. Peroxo linkage is simply a \[ - O - O - \] bond. It is also called a peroxo group or peroxide group. For example, Hydrogen peroxide, the most common peroxide has a peroxo linkage which is shown as \[ - H - O - O - H - \] . Here, the oxygen-oxygen bond is quite weak. The oxygen atoms in the peroxide ions have an oxidation state of \[ - 1\] . To know which of the options has a perexo linkage then we need to know the structure and molecular formula of every acid in the options. Let’s start with Sulphurous acid.
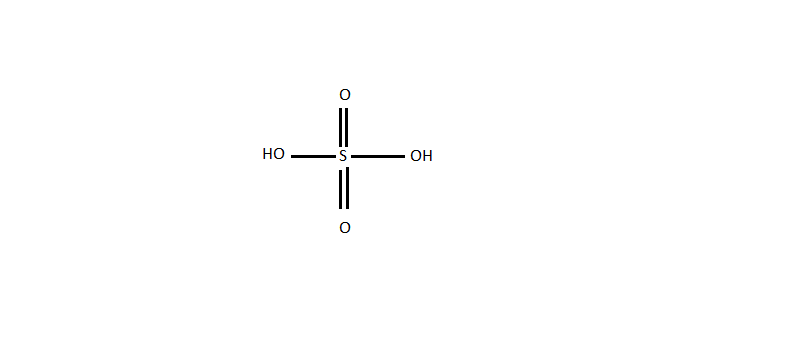
Sulphurous acid is also known as oil of vitriol. It is a mineral acid composed of elements sulphur, oxygen and hydrogen and has a molecular formula \[{H_2}S{O_4}\] . Its properties are colourless, odourless and viscous liquid and is soluble in water. It is synthesised in highly exothermic reactions. The structure of sulphuric acid is as follows:
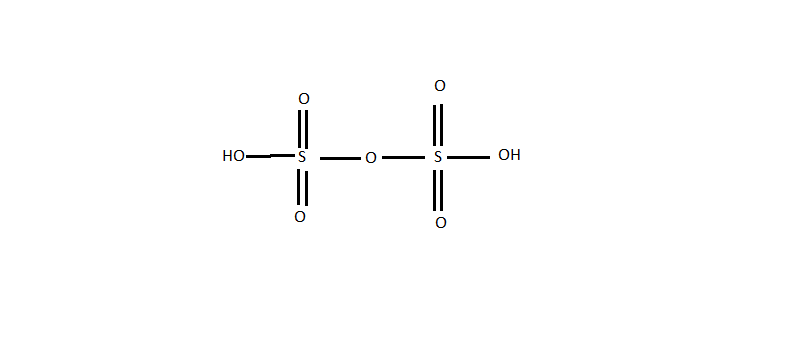
Pyrosulphuric acid is the main constituent of fuming sulphuric acid and is a strong acid. It is also known as disulphuric acid or oleum. It is weaker than sulphuric acid. Its molecular formula is \[{H_2}{S_2}{O_7}\] . It is used in the manufacture of explosives and dyes. It is also used as sulfonating agent and even have applications in petroleum refining. The structure is as given below.
Dithionic acid is also called Ethaebis, ethane dithionic acid and titration oxalic acid and has molecular formula \[{C_2}{H_2}{S_4}\] . It has structure as shown below.
Caro’s acid is also known as Persulphuric acid, Peroxy Sulfuric acid and Peroxymonosulphuric acid. Its molecular formula is \[{H_2}S{O_5}\] . Its structure is as given below.
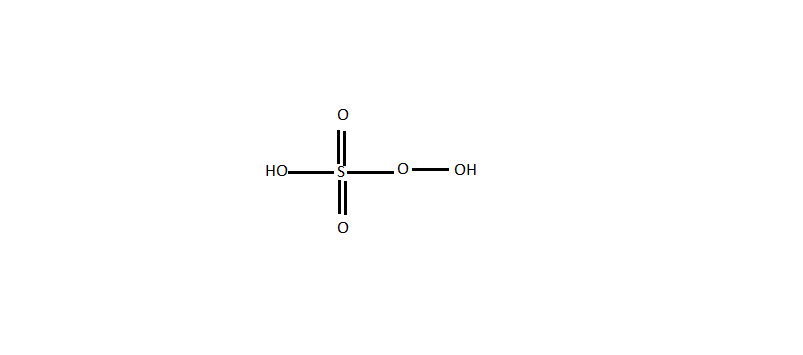
From the above structures of the four acids we get to know that the oxygen-oxygen bond is present only in Caro’s acid.
Therefore, the correct answer is option D.
Note: Knowing only one name of any compound is not enough. Questions can be asked using any name. Therefore, one should know every other name of every compound.
Step by step answer:
First, we need to know about perexo linkage. Peroxo linkage is simply a \[ - O - O - \] bond. It is also called a peroxo group or peroxide group. For example, Hydrogen peroxide, the most common peroxide has a peroxo linkage which is shown as \[ - H - O - O - H - \] . Here, the oxygen-oxygen bond is quite weak. The oxygen atoms in the peroxide ions have an oxidation state of \[ - 1\] . To know which of the options has a perexo linkage then we need to know the structure and molecular formula of every acid in the options. Let’s start with Sulphurous acid.
Sulphurous acid is also known as oil of vitriol. It is a mineral acid composed of elements sulphur, oxygen and hydrogen and has a molecular formula \[{H_2}S{O_4}\] . Its properties are colourless, odourless and viscous liquid and is soluble in water. It is synthesised in highly exothermic reactions. The structure of sulphuric acid is as follows:

Pyrosulphuric acid is the main constituent of fuming sulphuric acid and is a strong acid. It is also known as disulphuric acid or oleum. It is weaker than sulphuric acid. Its molecular formula is \[{H_2}{S_2}{O_7}\] . It is used in the manufacture of explosives and dyes. It is also used as sulfonating agent and even have applications in petroleum refining. The structure is as given below.

Dithionic acid is also called Ethaebis, ethane dithionic acid and titration oxalic acid and has molecular formula \[{C_2}{H_2}{S_4}\] . It has structure as shown below.

Caro’s acid is also known as Persulphuric acid, Peroxy Sulfuric acid and Peroxymonosulphuric acid. Its molecular formula is \[{H_2}S{O_5}\] . Its structure is as given below.

From the above structures of the four acids we get to know that the oxygen-oxygen bond is present only in Caro’s acid.
Therefore, the correct answer is option D.
Note: Knowing only one name of any compound is not enough. Questions can be asked using any name. Therefore, one should know every other name of every compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




