
The actual geometry of $N{{O}_{2}}$ is
(a) planar
(b) linear
(c) V-shape
(d) tetrahedral
Answer
533.7k+ views
Hint: Nitrogen has five electrons present in its valence shell and out of which three electrons exist as bond pairs and the remaining two electrons is present as lone pair. Now identify the hybridization of nitrogen dioxide and then you can easily find the geometry of nitrogen dioxide.
Complete answer:
First of let’s discuss what is hybridization. By the term hybridization we mean the phenomenon of intermixing of the orbitals of slightly different energies so as to redistribute their energies to give a new set of orbitals of equivalent energies and shape.
The conditions for the hybridization are as follows:
(i) only the orbitals present in the valence shell of the atom are hybridized.
(ii) the orbitals undergoing hybridization should have only a small energy difference. The orbitals which greatly differ in energies cannot take part in hybridization.
(iii) It is not necessary that only half-filled orbitals participate in hybridization. In certain cases, even half-filled orbitals of the valence shell participate in hybridization.
Now considering the statement as;
Nitrogen has atomic number as 7 and has five electrons in its outermost valence shell which are unpaired.
In nitrogen dioxide, nitrogen has five electrons in its valence shell. Out of which, two forms sigma bonds with the two oxygen atoms and third electron present in the p-orbital of nitrogen atom forms a pi-bond with the oxygen atom and the remaining two electrons is present as lone pair on the nitrogen atom. Thus, the hybridization of the molecule is $s{{p}^{3}}$ (two sigma bonds, one pi-bond and one lone pair).
The shape or geometry of nitrogen dioxide is:
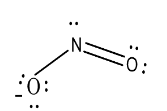
So, from this it is clear that the shape of nitrogen dioxide is bent V-shaped.
Hence, option (c) is correct.
Note: The hybridized orbitals are always equivalent in energy and shape and are more effective in forming stable bonds than the pure atomic orbitals .
Complete answer:
First of let’s discuss what is hybridization. By the term hybridization we mean the phenomenon of intermixing of the orbitals of slightly different energies so as to redistribute their energies to give a new set of orbitals of equivalent energies and shape.
The conditions for the hybridization are as follows:
(i) only the orbitals present in the valence shell of the atom are hybridized.
(ii) the orbitals undergoing hybridization should have only a small energy difference. The orbitals which greatly differ in energies cannot take part in hybridization.
(iii) It is not necessary that only half-filled orbitals participate in hybridization. In certain cases, even half-filled orbitals of the valence shell participate in hybridization.
Now considering the statement as;
Nitrogen has atomic number as 7 and has five electrons in its outermost valence shell which are unpaired.
In nitrogen dioxide, nitrogen has five electrons in its valence shell. Out of which, two forms sigma bonds with the two oxygen atoms and third electron present in the p-orbital of nitrogen atom forms a pi-bond with the oxygen atom and the remaining two electrons is present as lone pair on the nitrogen atom. Thus, the hybridization of the molecule is $s{{p}^{3}}$ (two sigma bonds, one pi-bond and one lone pair).
The shape or geometry of nitrogen dioxide is:

So, from this it is clear that the shape of nitrogen dioxide is bent V-shaped.
Hence, option (c) is correct.
Note: The hybridized orbitals are always equivalent in energy and shape and are more effective in forming stable bonds than the pure atomic orbitals .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




