
The amino ketone shown undergoes a spontaneous cyclization on standing. What is the major product of this intramolecular reaction?
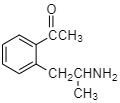
A.
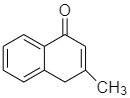
B.
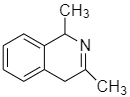
C.
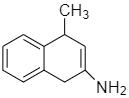
D.
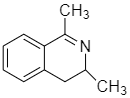





Answer
498k+ views
Hint: Organic compounds that carry an amine functional group, as well as ketone functional group, are known as Aminoketone and Cyclization is any reaction that brings about the formation of a ring.
A reaction between two or more atoms in the same reactant molecule is involved in an intramolecular reaction but covalency changes occur in two separate molecules in an intermolecular reaction. Intramolecular reaction particularly leads to the formation of 5 and 6 membered rings and are quick in contrast to an analogous intermolecular process.
Complete Step By Step Answer:
Step 1: Amino ketone undergoes intramolecular reaction and thus leading to cyclization when the electron-withdrawing draws the electron from the carbon atom (carbon atom that is attached to benzene and O) thereby making a carbocation which is further attacked by the lone pair of the nitrogen atom.

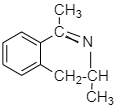
After the first step, the product obtained is shown in the given below figure.

Step 2: Now the hydrogen atom is released by $ NH_2^ \oplus $ while this released hydrogen atom is accepted by the $ {O^ - } $ atom forming $ OH $ .
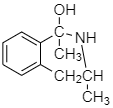
The product obtained from step 2 is as follows:
Now, this product again repeats step 1 leading to the formation of
Therefore the correct answer is option D.
Note:
For 3 and 4 members, at the transition state, the slow rate is an outcome of angle strain. Even if 3 membered rings are more strained, aziridine formation is faster than azetidine formation because of the proximity of the nucleophile and leaving group that increases the probability to meet in a reactive conformation.
A reaction between two or more atoms in the same reactant molecule is involved in an intramolecular reaction but covalency changes occur in two separate molecules in an intermolecular reaction. Intramolecular reaction particularly leads to the formation of 5 and 6 membered rings and are quick in contrast to an analogous intermolecular process.
Complete Step By Step Answer:
Step 1: Amino ketone undergoes intramolecular reaction and thus leading to cyclization when the electron-withdrawing draws the electron from the carbon atom (carbon atom that is attached to benzene and O) thereby making a carbocation which is further attacked by the lone pair of the nitrogen atom.

After the first step, the product obtained is shown in the given below figure.

Step 2: Now the hydrogen atom is released by $ NH_2^ \oplus $ while this released hydrogen atom is accepted by the $ {O^ - } $ atom forming $ OH $ .
The product obtained from step 2 is as follows:

Now, this product again repeats step 1 leading to the formation of

Therefore the correct answer is option D.
Note:
For 3 and 4 members, at the transition state, the slow rate is an outcome of angle strain. Even if 3 membered rings are more strained, aziridine formation is faster than azetidine formation because of the proximity of the nucleophile and leaving group that increases the probability to meet in a reactive conformation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




