
The angle $\angle \text{R-O-R}$ is:
A) ${{115}^{0}}$
B) ${{95}^{0}}$
C) ${{110}^{0}}$
D) ${{105}^{0}}$
Answer
587.7k+ views
Hint: In Ether, the oxygen atom is higher in electronegativity. The carbon-oxygen bond is polar. The bulkier alkyl groups in ether push out each other due to steric repulsion. Thus the bond angle $\angle \text{R-O-R}$ is on the higher end.
Complete answer:
Ethers are the organic molecule having a general structure as:
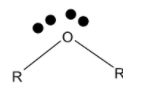
> Ethers have an oxygen atom bonded to the two alkyl groups opposite to each other. If we consider the oxygen as the centre of the molecule, then it holds two alkyl groups and two lone pairs of electrons on it. Thus in ethers, the oxygen atom is $s{{p}^{3}}$ hybridized. The ether exists in a tetrahedral geometry. It is expected that the two lone pairs and two alkyl groups are at the apexes of the tetrahedron.
> Let us compare it with the simple $s{{p}^{3}}$ hybridized molecule methane. In methane, the four substituents are at the corners of the tetrahedron. The bond angle between the atoms i.e. $\angle \text{H-C-H}$ is found to be ${{109}^{0}}5'$.
> Thus if the ether has the tetrahedral geometry then the bond angle $\angle \text{R-O-R}$ should be equal to ${{109}^{0}}$. However, the bond angle is slightly greater than the ${{109}^{0}}$.
> The oxygen has a greater electronegativity than carbon. Thus the oxygen withdraws the electron density from alkyl ($-\text{R}$) groups. The $\text{C-O}$ bond becomes slightly polar.
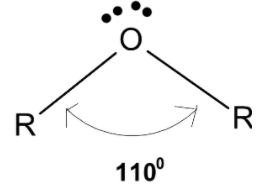
> The polar bond is inclined to each other at the angle of ${{110}^{0}}$ and results in the net dipole moment. The bond angle is greater than that of the tetrahedral bond because the internal repulsion due to the larger hydrocarbon part which is due to $-\text{R}$ groups on the oxygen is greater than the external repulsion experienced by the lone pair of oxygen. Ideally, the lone pair should contract the bond angle but the bond expands due to steric repulsion of groups.
Thus the bond angle between the alkyl and oxygen atoms $\angle \text{R-O-R}$ in ether is ${{110}^{0}}$.
Hence, (C) is the correct option.
Note: It is known that lone pair-bond pair repulsion is maximum. Here in ethers the repulsion due to the large, bulkier hydrocarbons is considered to be the main reason behind the expansion of angle.
Complete answer:
Ethers are the organic molecule having a general structure as:

> Ethers have an oxygen atom bonded to the two alkyl groups opposite to each other. If we consider the oxygen as the centre of the molecule, then it holds two alkyl groups and two lone pairs of electrons on it. Thus in ethers, the oxygen atom is $s{{p}^{3}}$ hybridized. The ether exists in a tetrahedral geometry. It is expected that the two lone pairs and two alkyl groups are at the apexes of the tetrahedron.
> Let us compare it with the simple $s{{p}^{3}}$ hybridized molecule methane. In methane, the four substituents are at the corners of the tetrahedron. The bond angle between the atoms i.e. $\angle \text{H-C-H}$ is found to be ${{109}^{0}}5'$.
> Thus if the ether has the tetrahedral geometry then the bond angle $\angle \text{R-O-R}$ should be equal to ${{109}^{0}}$. However, the bond angle is slightly greater than the ${{109}^{0}}$.
> The oxygen has a greater electronegativity than carbon. Thus the oxygen withdraws the electron density from alkyl ($-\text{R}$) groups. The $\text{C-O}$ bond becomes slightly polar.

> The polar bond is inclined to each other at the angle of ${{110}^{0}}$ and results in the net dipole moment. The bond angle is greater than that of the tetrahedral bond because the internal repulsion due to the larger hydrocarbon part which is due to $-\text{R}$ groups on the oxygen is greater than the external repulsion experienced by the lone pair of oxygen. Ideally, the lone pair should contract the bond angle but the bond expands due to steric repulsion of groups.
Thus the bond angle between the alkyl and oxygen atoms $\angle \text{R-O-R}$ in ether is ${{110}^{0}}$.
Hence, (C) is the correct option.
Note: It is known that lone pair-bond pair repulsion is maximum. Here in ethers the repulsion due to the large, bulkier hydrocarbons is considered to be the main reason behind the expansion of angle.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




