
The atomic number of fluorine is 9. Explain the formation of the fluoride ion.
Answer
588k+ views
Hint: Consider the atomic number of fluorine and then formulate the possible electronic configuration of the atom. From this we can deduce how the fluoride ion will be formed.
Complete step by step answer:
The chemical representation of the fluoride ion is ${{F}^{-}}$. It has 1 electron extra as compared to the number of protons present in its nucleus.
We know that the atomic number of fluorine is 9 and the overall electronic configuration is 2, 7. The first orbital contains 2 electrons and the second orbital contains 7 electrons. 8 electrons are required in the second orbital to form a stable octet. The location of fluorine in the periodic table is:
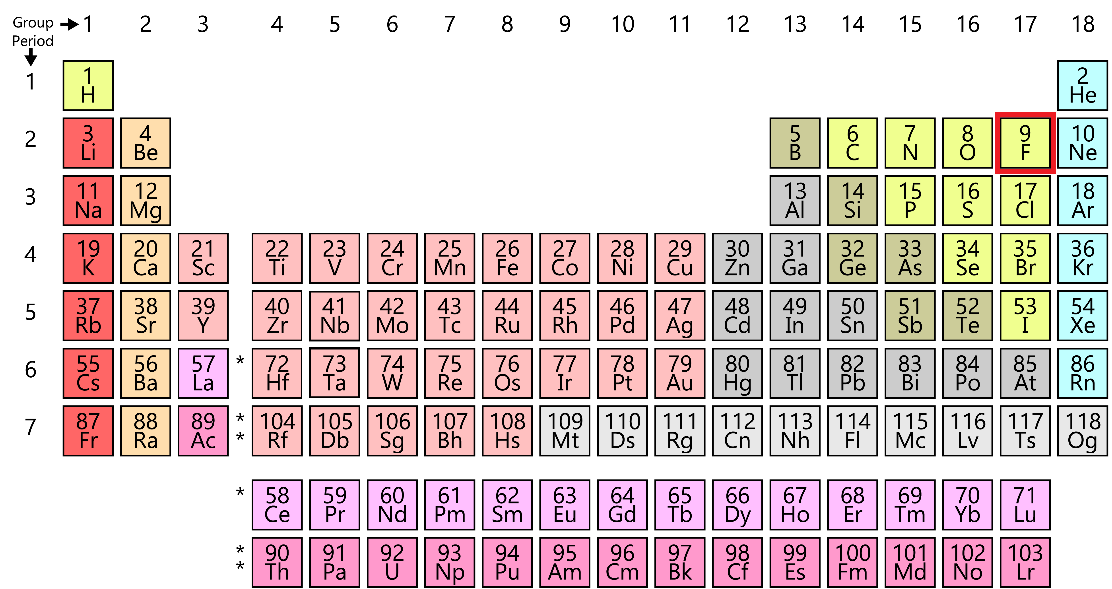
Here, we can see that fluorine is present in the seventeenth group of the periodic table. We have already seen that fluorine requires one electron to form a stable octet. So, it takes one electron from any surrounding atom and completes its octet. The general reaction for the formation of the fluoride ion is:
\[{{F}_{2}}+2{{e}^{-}}\to 2{{F}^{-}}\]
Since, the fluoride ion is far more stable than the fluorine atom, energy is released while the formation of the fluoride ion. And the reaction that takes place is usually exothermic.
Note: Remember that fluorine is the most electronegative element and has an electronegativity of 4.0 on Pauling’s Scale of electronegativity. Thus, the phenomenon of the formation of a ${{F}^{+}}$ ion will never happen since none of the elements are electronegative enough to take an electron from fluorine.
Complete step by step answer:
The chemical representation of the fluoride ion is ${{F}^{-}}$. It has 1 electron extra as compared to the number of protons present in its nucleus.
We know that the atomic number of fluorine is 9 and the overall electronic configuration is 2, 7. The first orbital contains 2 electrons and the second orbital contains 7 electrons. 8 electrons are required in the second orbital to form a stable octet. The location of fluorine in the periodic table is:

Here, we can see that fluorine is present in the seventeenth group of the periodic table. We have already seen that fluorine requires one electron to form a stable octet. So, it takes one electron from any surrounding atom and completes its octet. The general reaction for the formation of the fluoride ion is:
\[{{F}_{2}}+2{{e}^{-}}\to 2{{F}^{-}}\]
Since, the fluoride ion is far more stable than the fluorine atom, energy is released while the formation of the fluoride ion. And the reaction that takes place is usually exothermic.
Note: Remember that fluorine is the most electronegative element and has an electronegativity of 4.0 on Pauling’s Scale of electronegativity. Thus, the phenomenon of the formation of a ${{F}^{+}}$ ion will never happen since none of the elements are electronegative enough to take an electron from fluorine.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




