
The atomic number of Sn is 50. The shape of gaseous $SnC{{l}_{2}}$ molecule is:
(a)- $Cl-Sn-Cl$
(b)-
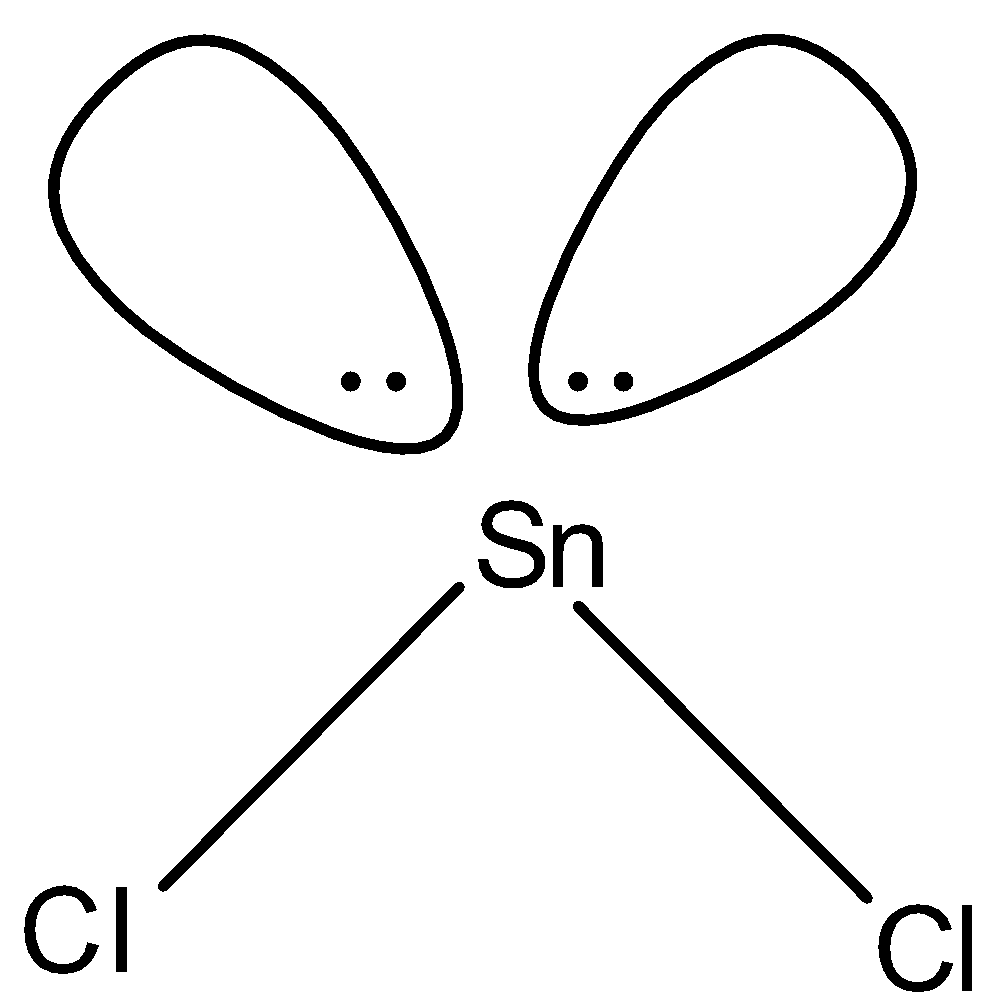
(c)-
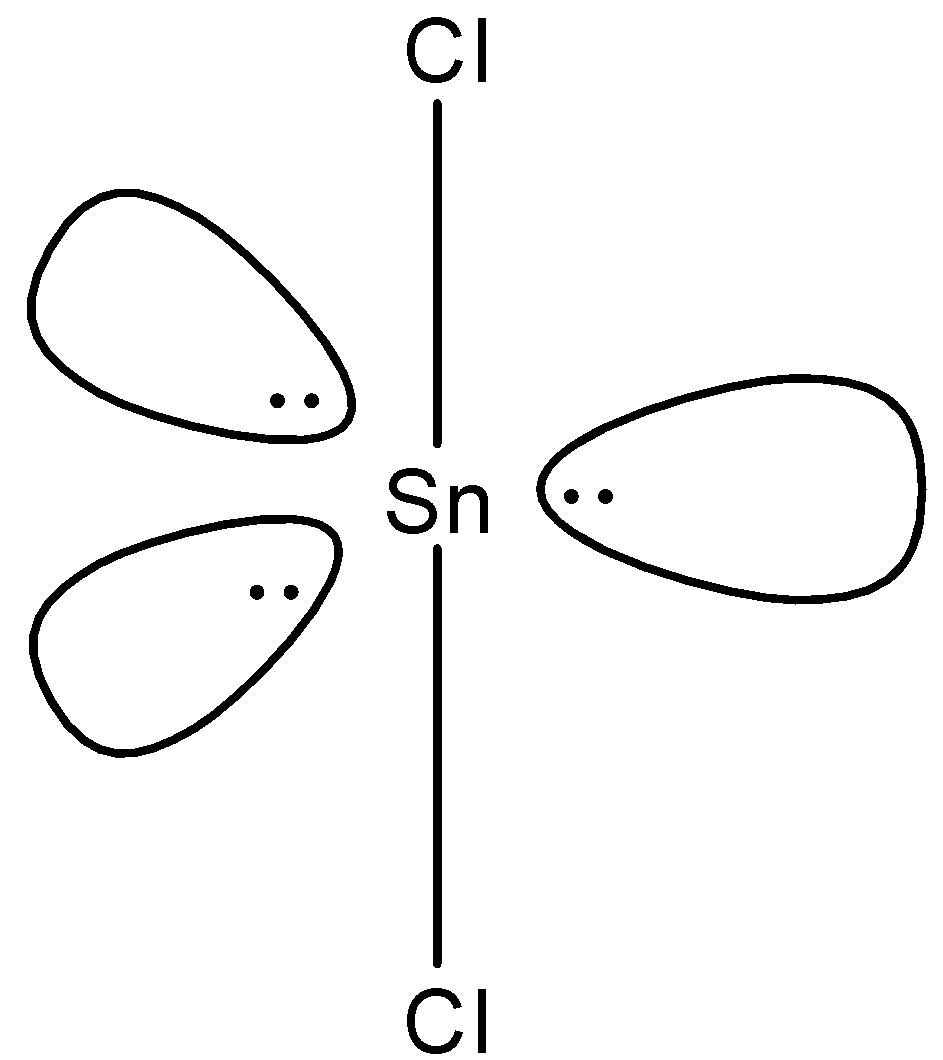
(d)-
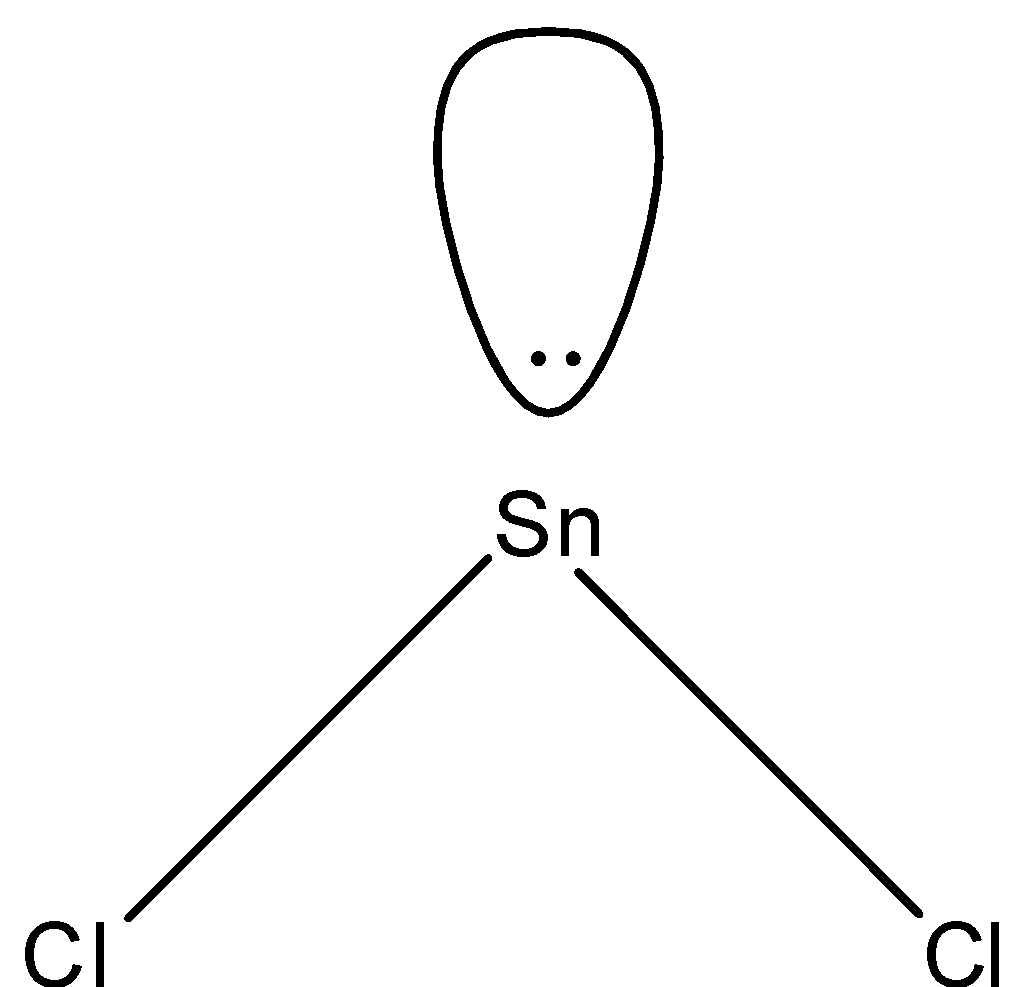



Answer
569.4k+ views
Hint: The atomic number is 50 means there are 50 electrons in the atom and they will be filled according to the order: $\text{1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p}$. The number of electrons in the last shell will decide the shape of the molecule. In $SnC{{l}_{2}}$, two electrons of tin will be shared by chlorine and the rest will be the lone pairs.
Complete Solution :
We are given the atomic number of Sn is 50. The atomic number is 50 means there are 50 electrons in the atom and they will be filled according to the order: $\text{1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p}$. We know that the s can accommodate 2 electrons, p can accommodate 6 electrons, d can accommodate 10 electrons, and f can accommodate 14 electrons. So, the configuration will be:
$\text{1}{{\text{s}}^{2}}\text{ 2}{{\text{s}}^{2}}\text{ 2}{{\text{p}}^{6}}\text{ 3}{{\text{s}}^{2}}\text{ 3}{{\text{p}}^{6}}\text{ 4}{{\text{s}}^{2}}\text{ 3}{{\text{d}}^{10}}\text{ 4}{{\text{p}}^{6}}\text{ 5}{{\text{s}}^{2}}\text{ 4}{{\text{d}}^{10}}\text{ 5}{{\text{p}}^{2}}$
- So, the last orbital is 5s and 5p, hence there are 4 electrons in its outermost shell. Therefore, we can say that the Sn (tin) is the element of group 14 (carbon family).
- In $SnC{{l}_{2}}$, two electrons of tin will be shared by chlorine and the rest will be the lone pairs. Since we have 4 electrons in its outermost shell, 2 will be bonded with chlorine and there will be one lone pair and the shape of the molecule will be bent-shape. The structure is given below:
So, the correct answer is “Option D”.
Note: We know that in a compound having three atoms, one central atom and two same atoms joining the central atom, the shape is always linear but the $SnC{{l}_{2}}$ is bent-shape because of the presence of lone pair which causes the repulsion.
Complete Solution :
We are given the atomic number of Sn is 50. The atomic number is 50 means there are 50 electrons in the atom and they will be filled according to the order: $\text{1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p}$. We know that the s can accommodate 2 electrons, p can accommodate 6 electrons, d can accommodate 10 electrons, and f can accommodate 14 electrons. So, the configuration will be:
$\text{1}{{\text{s}}^{2}}\text{ 2}{{\text{s}}^{2}}\text{ 2}{{\text{p}}^{6}}\text{ 3}{{\text{s}}^{2}}\text{ 3}{{\text{p}}^{6}}\text{ 4}{{\text{s}}^{2}}\text{ 3}{{\text{d}}^{10}}\text{ 4}{{\text{p}}^{6}}\text{ 5}{{\text{s}}^{2}}\text{ 4}{{\text{d}}^{10}}\text{ 5}{{\text{p}}^{2}}$
- So, the last orbital is 5s and 5p, hence there are 4 electrons in its outermost shell. Therefore, we can say that the Sn (tin) is the element of group 14 (carbon family).
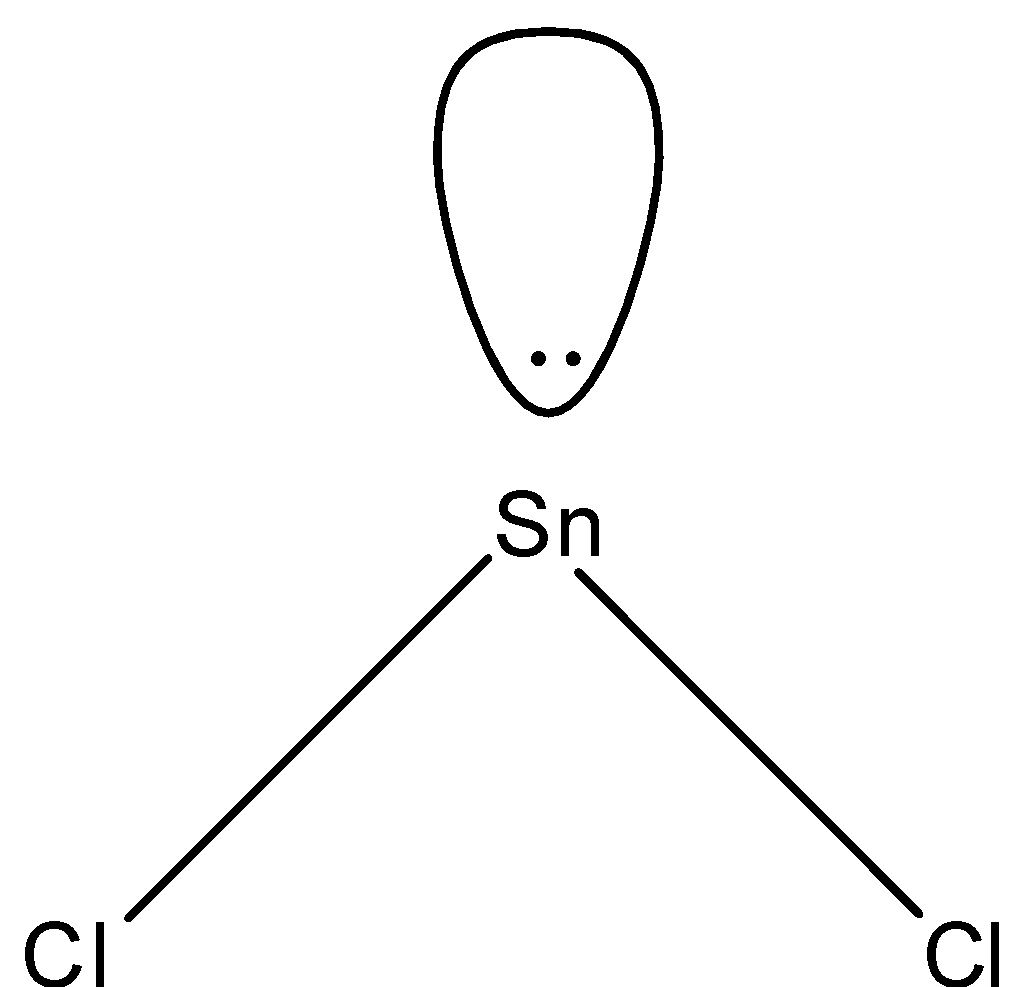
- In $SnC{{l}_{2}}$, two electrons of tin will be shared by chlorine and the rest will be the lone pairs. Since we have 4 electrons in its outermost shell, 2 will be bonded with chlorine and there will be one lone pair and the shape of the molecule will be bent-shape. The structure is given below:

So, the correct answer is “Option D”.
Note: We know that in a compound having three atoms, one central atom and two same atoms joining the central atom, the shape is always linear but the $SnC{{l}_{2}}$ is bent-shape because of the presence of lone pair which causes the repulsion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




