
The behavior of temporary gases like carbon dioxide approaches that of permanent gases such as nitrogen, oxygen etc.., as we go?
A.Below critical temperature
B.Above critical temperature
C.Above absolute zero
D.Below absolute zero
Answer
594k+ views
Hint:Temporary gases have strong intermolecular forces and attraction. So, they can be easily liquefied and hence, critical temperature would be higher. Permanent gas is a gas believed to be incapable of liquefaction. They remain in constant relative quantities over time.
Complete step by step answer:
-The critical temperature is the measures to identify the strength of inter- molecular forces of attraction. It is the temperature at and above which vapor of the substance cannot be liquefied, no matter how much pressure is applied. Every substance has a critical temperature.
-Temporary gases have strong intermolecular forces and attractions. So, they can be easily liquefied and hence, critical temperature would be higher.
-Thus, above critical temperature, the liquefaction behavior of temporary gases approaches that of permanent gases.
-Liquefaction of gas occurs when the intermolecular forces of attraction reach a point that they become capable to bind the gas molecules together to convert it into a liquid state
-Higher the critical temperature, faster is the liquefaction of the gas.
We have a graph below which highlights critical temperature and critical pressure. Let’s have a look.
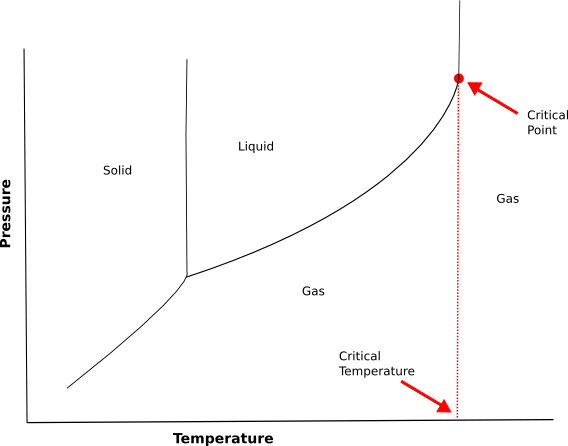
-The liquid gas line slopes up to the right. One way to think about this is that as molecules move faster , the attractions they have for each other becomes less effective .But if the gas is compressed enough, the molecules will bump into each other so often that they will eventually start to stick together and become a liquid.
-However, if the temperature gets high enough, the particles are moving so fast, that they will act independently even if they are packed tightly together. This temperature is called critical temperature.
-At that temperature, the liquid gas line becomes, essentially, a vertical line with all areas to the right of that line occupied by the gas phase.
-The point on the graph where the liquid gas line reaches the critical temperature and becomes a vertical line is called critical point and the temperature at which this happens is called the critical temperature.
Hence, option B is correct.
Additional information:
A greenhouse gas is a gas that absorbs and emits radiant energy within the thermal infrared range.. There are several greenhouse gases responsible for warming, and humans emit them in a variety of ways. Most come from the combustion of fossil fuels in cars, buildings, factories and power points. The gas responsible for the most warming is carbon dioxide.
The concentration of nitrogen is the highest in our environment. It accounts for about 78% of the Earth’s atmosphere. The next is oxygen which constitutes 20.95%, then comes carbon dioxide which is 0.0360%, and last is hydrogen 0.00005%.
Note:
Above critical temperature, it is difficult to liquefy gases because the kinetic energy becomes sufficiently high and gases cannot be liquefied no matter what pressure we apply.
Complete step by step answer:
-The critical temperature is the measures to identify the strength of inter- molecular forces of attraction. It is the temperature at and above which vapor of the substance cannot be liquefied, no matter how much pressure is applied. Every substance has a critical temperature.
-Temporary gases have strong intermolecular forces and attractions. So, they can be easily liquefied and hence, critical temperature would be higher.
-Thus, above critical temperature, the liquefaction behavior of temporary gases approaches that of permanent gases.
-Liquefaction of gas occurs when the intermolecular forces of attraction reach a point that they become capable to bind the gas molecules together to convert it into a liquid state
-Higher the critical temperature, faster is the liquefaction of the gas.
We have a graph below which highlights critical temperature and critical pressure. Let’s have a look.

-The liquid gas line slopes up to the right. One way to think about this is that as molecules move faster , the attractions they have for each other becomes less effective .But if the gas is compressed enough, the molecules will bump into each other so often that they will eventually start to stick together and become a liquid.
-However, if the temperature gets high enough, the particles are moving so fast, that they will act independently even if they are packed tightly together. This temperature is called critical temperature.
-At that temperature, the liquid gas line becomes, essentially, a vertical line with all areas to the right of that line occupied by the gas phase.
-The point on the graph where the liquid gas line reaches the critical temperature and becomes a vertical line is called critical point and the temperature at which this happens is called the critical temperature.
Hence, option B is correct.
Additional information:
A greenhouse gas is a gas that absorbs and emits radiant energy within the thermal infrared range.. There are several greenhouse gases responsible for warming, and humans emit them in a variety of ways. Most come from the combustion of fossil fuels in cars, buildings, factories and power points. The gas responsible for the most warming is carbon dioxide.
The concentration of nitrogen is the highest in our environment. It accounts for about 78% of the Earth’s atmosphere. The next is oxygen which constitutes 20.95%, then comes carbon dioxide which is 0.0360%, and last is hydrogen 0.00005%.
Note:
Above critical temperature, it is difficult to liquefy gases because the kinetic energy becomes sufficiently high and gases cannot be liquefied no matter what pressure we apply.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




