
The best way to prepare polyisobutylene is:
A.Coordination polymerization
B.Free radical polymerization
C.Cationic polymerization
D.Anionic polymerization
Answer
581.1k+ views
Hint:Cationic polymerization is performed to produce oligomers and high polymers such as polyisobutylene. It is a type of chain growth polymerisation in which cationic initiator transfers charge to monomer which when becomes reactive polyisobutylene.
Complete step by step answer:
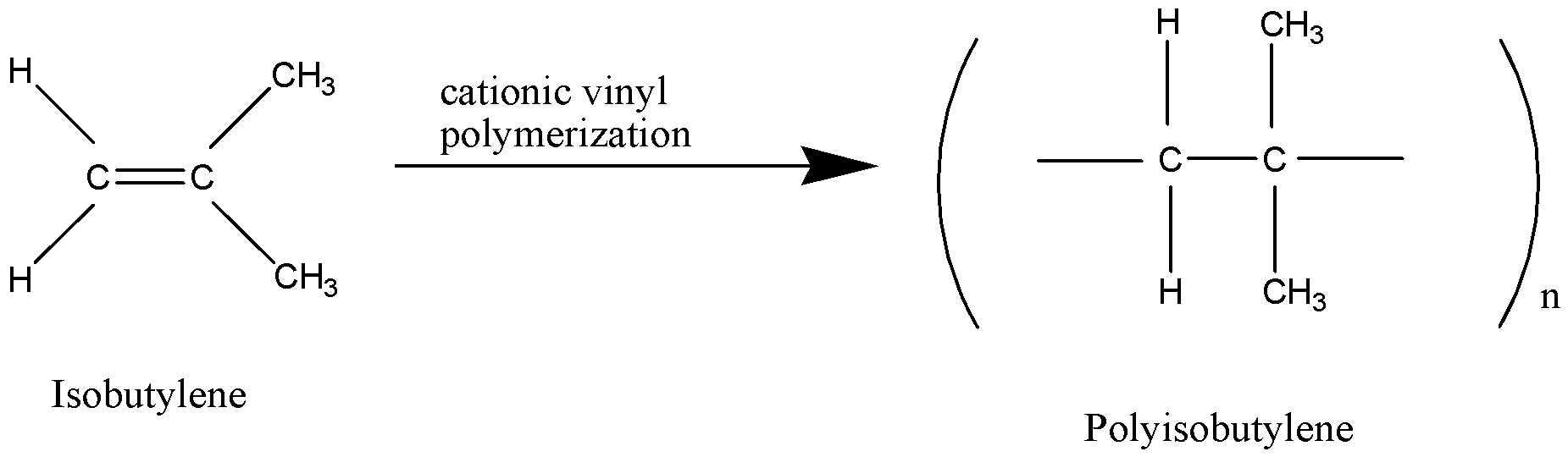
Polyisobutylene is a synthetic polymer produced by the low-temperature polymerization of isobutylene in liquid ethylene, methylene chloride, or hexane, using an aluminium-chloride or boron-trifluoride catalyst. It may contain a suitable stabilizer. It is a very versatile, non-toxic, water-white viscous liquid.
Cationic polymerization is a way to make polymers from small molecules, or monomers which contain carbon – carbon double bond. Its primary commercial use is for making polyisobutylene. In cationic polymerization, the cation is stabilised by 8 \[\alpha -H\] and +I effect and hence, the cationic polymerization is the best way to prepare polyisobutylene.
Cationic polymerization is performed to produce oligomers and high polymers of considerable technological importance.
Cationic polymerization depends on the use of cationic initiators which includes reagents capable of providing positive ions or H+ ions. They are effective with monomers containing electron releasing groups like methyl or phenyl etc.
Polyisobutylene ${({C_4}{H_8})_n}$ is a homo polymer of isobutylene on which butyl rubber is based. It is prepared by cationic polymerization.
Hence option C is correct.
Note:
Polyisobutylene is a synthetic rubber, or elastomer. It is the only rubber that is gas impermeable. It is sometimes known as butyl rubber. It is preserved in well-closed containers and no storage requirements specified. Because polyisobutylene will hold air, it is used to make things like the inner liner of tires and basketballs. Polyisobutylene is primarily used in the manufacture of inner tubes of tires and its other applications may include adhesives, agricultural chemicals, caulks, sealants, paper & pulp, and chewing gum. Polyisobutylene was the first developed during the early 1940s.
Complete step by step answer:
Polyisobutylene is a synthetic polymer produced by the low-temperature polymerization of isobutylene in liquid ethylene, methylene chloride, or hexane, using an aluminium-chloride or boron-trifluoride catalyst. It may contain a suitable stabilizer. It is a very versatile, non-toxic, water-white viscous liquid.
Cationic polymerization is a way to make polymers from small molecules, or monomers which contain carbon – carbon double bond. Its primary commercial use is for making polyisobutylene. In cationic polymerization, the cation is stabilised by 8 \[\alpha -H\] and +I effect and hence, the cationic polymerization is the best way to prepare polyisobutylene.
Cationic polymerization is performed to produce oligomers and high polymers of considerable technological importance.
Cationic polymerization depends on the use of cationic initiators which includes reagents capable of providing positive ions or H+ ions. They are effective with monomers containing electron releasing groups like methyl or phenyl etc.
Polyisobutylene ${({C_4}{H_8})_n}$ is a homo polymer of isobutylene on which butyl rubber is based. It is prepared by cationic polymerization.

Hence option C is correct.
Note:
Polyisobutylene is a synthetic rubber, or elastomer. It is the only rubber that is gas impermeable. It is sometimes known as butyl rubber. It is preserved in well-closed containers and no storage requirements specified. Because polyisobutylene will hold air, it is used to make things like the inner liner of tires and basketballs. Polyisobutylene is primarily used in the manufacture of inner tubes of tires and its other applications may include adhesives, agricultural chemicals, caulks, sealants, paper & pulp, and chewing gum. Polyisobutylene was the first developed during the early 1940s.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




