
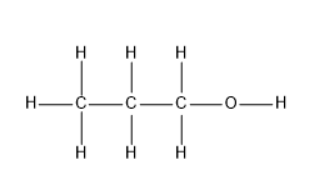
The bond between which two atoms has the greatest degree of polarity in the following molecule?

Answer
493.5k+ views
Hint: Given molecule is propanol which is an alcohol. The oxygen is more electronegative compared to the carbon and hydrogen atoms. Carbon is more electronegative compared to hydrogen. Thus, based on the electronegativities the oxygen hydrogen bond is more polar in the given molecule.
Complete Step By Step Answer:
Electronegativity is defined as the tendency of attraction of a shared pair of electrons towards itself. In the periodic table halogens are the most electronegative elements. Oxygen is an electronegative element less than halogens.
According to Pauling electronegativity scale, the value of electronegativity of oxygen is $ 3.44 $ and hydrogen has $ 2.20 $ and carbon has the electronegativity of $ 2.55 $
If there is more difference in the electronegativity values of atoms, more will be the polarity of the bond, as one atom will attract the electrons from the other atom leads to the polarity or movement of electrons.
The given molecule is propanol which has carbon, oxygen and hydrogen atoms. Out of these three atoms, oxygen has more electronegativity and hydrogen has least electronegativity. From this the oxygen-hydrogen bond is polar.
Thus, the bond between oxygen and hydrogen atoms has the greatest degree of polarity in the given molecule.
Note:
Due to the presence of oxygen and hydrogen bonds in water, water is considered as a high polar solvent. Alcohols are the chemical compounds that consist of oxygen hydrogen bonds known as polar compounds. Due to the presence of $ O - H $ bond alcohols will be soluble in water.
Complete Step By Step Answer:
Electronegativity is defined as the tendency of attraction of a shared pair of electrons towards itself. In the periodic table halogens are the most electronegative elements. Oxygen is an electronegative element less than halogens.
According to Pauling electronegativity scale, the value of electronegativity of oxygen is $ 3.44 $ and hydrogen has $ 2.20 $ and carbon has the electronegativity of $ 2.55 $
If there is more difference in the electronegativity values of atoms, more will be the polarity of the bond, as one atom will attract the electrons from the other atom leads to the polarity or movement of electrons.
The given molecule is propanol which has carbon, oxygen and hydrogen atoms. Out of these three atoms, oxygen has more electronegativity and hydrogen has least electronegativity. From this the oxygen-hydrogen bond is polar.
Thus, the bond between oxygen and hydrogen atoms has the greatest degree of polarity in the given molecule.
Note:
Due to the presence of oxygen and hydrogen bonds in water, water is considered as a high polar solvent. Alcohols are the chemical compounds that consist of oxygen hydrogen bonds known as polar compounds. Due to the presence of $ O - H $ bond alcohols will be soluble in water.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




