
The bonding in ammonium chloride:
A: Is covalent only
B: Is electrovalent only
C: Consists of three covalent nitrogen- hydrogen bonds and an electrovalent bond between the ammonia molecule and the chlorine atom
D: Consists of four covalent nitrogen- hydrogen bonds and an electrovalent bond between the ammonia molecule and the chlorine atom
Answer
559.2k+ views
Hint: The bonding is associated with the transfer or sharing of electrons between the atomic centres of the participating atoms and relies on the electrostatic attraction between the protons in the nuclei of both the atoms and the electrons in their orbitals. This is the force which holds the atoms together in the molecules.
Complete step by step answer:
Ammonium chloride with the chemical formula of $N{H_4}Cl$ is an organic compound. It is a white crystalline salt which is highly soluble in water while it’s own solutions are mildly acidic. We know that, Ammonium chloride is formed by the reaction of hydrochloric acid HCl with ammonia $N{H_3}$ This reaction is can be written as:
$N{H_3} + HCl \to N{H_4}Cl$
The structure of Ammonium chloride is shown below:
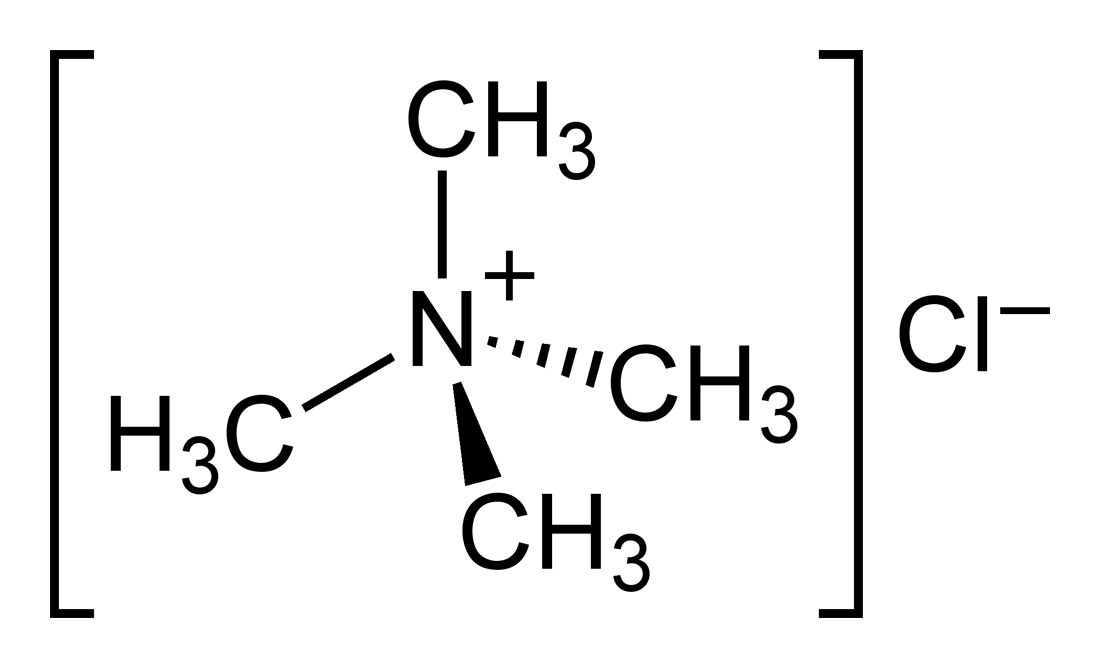
From the structure of ammonium chloride, it can be clearly seen that there are four covalent bonds which do exist between the nitrogen atom and the four hydrogen atoms. But, the option says that ammonium chloride contains the covalent bond only which is not true.
In the structure of ammonium chloride, the electrovalent bond can be seen between the ammonium ion $N{H_4}^ + $ and the chloride ion $C{l^ - }$. But it is not true that the ammonium chloride contains only electrovalent bonds. So, this option is also not true.
As we can clearly see from the structure of the ammonium chloride that there are four covalent bonds that are existing between the nitrogen atom and the four hydrogen atoms. Moreover, the electrovalent bond exists between ammonium ion and the chloride ion that is not between the ammonium molecule and chlorine ion. So, this option is also not true.
As we have been talking so far, the four covalent bonds and one electrovalent bond between nitrogen and hydrogen atoms and between ammonium ion and chloride ion respectively, can be seen in the structure of ammonium chloride.
So, the correct answer is Option D.
Note: In covalent bond, the electrons are mutually shared between the atoms involved in the bonding whereas in the electrovalent bond, linkage is due to the force of electrostatic attraction.
Complete step by step answer:
Ammonium chloride with the chemical formula of $N{H_4}Cl$ is an organic compound. It is a white crystalline salt which is highly soluble in water while it’s own solutions are mildly acidic. We know that, Ammonium chloride is formed by the reaction of hydrochloric acid HCl with ammonia $N{H_3}$ This reaction is can be written as:
$N{H_3} + HCl \to N{H_4}Cl$
The structure of Ammonium chloride is shown below:

From the structure of ammonium chloride, it can be clearly seen that there are four covalent bonds which do exist between the nitrogen atom and the four hydrogen atoms. But, the option says that ammonium chloride contains the covalent bond only which is not true.
In the structure of ammonium chloride, the electrovalent bond can be seen between the ammonium ion $N{H_4}^ + $ and the chloride ion $C{l^ - }$. But it is not true that the ammonium chloride contains only electrovalent bonds. So, this option is also not true.
As we can clearly see from the structure of the ammonium chloride that there are four covalent bonds that are existing between the nitrogen atom and the four hydrogen atoms. Moreover, the electrovalent bond exists between ammonium ion and the chloride ion that is not between the ammonium molecule and chlorine ion. So, this option is also not true.
As we have been talking so far, the four covalent bonds and one electrovalent bond between nitrogen and hydrogen atoms and between ammonium ion and chloride ion respectively, can be seen in the structure of ammonium chloride.
So, the correct answer is Option D.
Note: In covalent bond, the electrons are mutually shared between the atoms involved in the bonding whereas in the electrovalent bond, linkage is due to the force of electrostatic attraction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




