
The Carbon dioxide molecule contains how many double bonds?
Answer
534k+ views
Hint: Carbon dioxide is a colorless gas with a density which is around $ 53\% $ than that of dry air, Carbon dioxide molecules consist of a carbon atom covalently double bonded to two oxygen atoms, It’s bond length is about $ 116.3pm $ and has a linear structure. Its linear structure is supported by an experimentally determined dipole moment of $ 0D $ .
Complete answer:
The Carbon dioxide molecule $ C{O_2} $ , has two double bonds which is illustrated in the figure below:
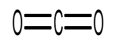
From the structure it is clear that carbon dioxide has $ 2 $ double bonds with a bond length of $ 116.3pm $ .
$ C{O_2} $ has a contribution of $ 0.04\% $ by volume in the atmosphere, It is an odorless gas with a trigonal crystal structure in solid state and a linear shape. It was discovered by Flemish Chemist Jan Baptist van Helmont.
Carbon dioxide is produced by all aerobic organisms when they metabolize organic compounds present in their diet to produce energy by respiration. It is obtained during decay of organic materials and fermentation of sugars. Carbon dioxide is the most long lived greenhouse gas in the atmosphere and is the major cause of ocean acidification because it dissolves in water to form carbonic acid.
Note: $ C{O_2} $ is used in fire extinguishers and is widely used in making soda glass. It has a melting point of around $ - 56.6^\circ C $ and a molar mass of $ 44.009{\raise0.5ex\hbox{ $ \scriptstyle g $ }
\kern-0.1em/\kern-0.15em \lower0.25ex\hbox{ $ \scriptstyle {mole} $ }} $ . It has a refractive index of around $ 1.00045 $ . In solid state it is known as Dry Ice and is used as a refrigerant in this state. It is also used as an additive in carbonic beverages. It was first liquefied by Sir Humphry Davy and Sir Michael Faraday.
Complete answer:
The Carbon dioxide molecule $ C{O_2} $ , has two double bonds which is illustrated in the figure below:

From the structure it is clear that carbon dioxide has $ 2 $ double bonds with a bond length of $ 116.3pm $ .
$ C{O_2} $ has a contribution of $ 0.04\% $ by volume in the atmosphere, It is an odorless gas with a trigonal crystal structure in solid state and a linear shape. It was discovered by Flemish Chemist Jan Baptist van Helmont.
Carbon dioxide is produced by all aerobic organisms when they metabolize organic compounds present in their diet to produce energy by respiration. It is obtained during decay of organic materials and fermentation of sugars. Carbon dioxide is the most long lived greenhouse gas in the atmosphere and is the major cause of ocean acidification because it dissolves in water to form carbonic acid.
Note: $ C{O_2} $ is used in fire extinguishers and is widely used in making soda glass. It has a melting point of around $ - 56.6^\circ C $ and a molar mass of $ 44.009{\raise0.5ex\hbox{ $ \scriptstyle g $ }
\kern-0.1em/\kern-0.15em \lower0.25ex\hbox{ $ \scriptstyle {mole} $ }} $ . It has a refractive index of around $ 1.00045 $ . In solid state it is known as Dry Ice and is used as a refrigerant in this state. It is also used as an additive in carbonic beverages. It was first liquefied by Sir Humphry Davy and Sir Michael Faraday.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




