
The colour developed when sodium sulphide is added to sodium nitroprusside is:
A. purple
B. yellow
C. red
D. black
Answer
590.7k+ views
Hint: When a chemical reaction occurs, one of the signs of reaction completion is change in colour. Colour change is observed when $\text{N}{{\text{a}}_{2}}\text{S}$ reacts with sodium and nitrogenous compound that is nitroprusside, whose formula is $\text{N}{{\text{a}}_{2}}\left[ \text{Fe}{{\left( \text{CN} \right)}_{5}}\text{NO} \right]$.
Complete answer:
Let us talk about sodium nitroprusside first to understand this compound.
It is a complex red-coloured compound that has an octahedral structure with iron(III) at the centre and surrounded by one linear nitric oxide ligand and five cyanide ligands. The structure of sodium nitroprusside is:
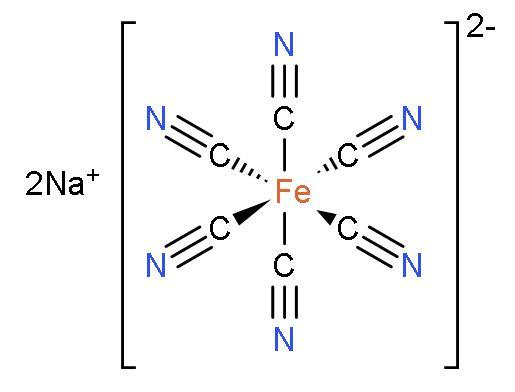
Sodium sulphide or $\text{N}{{\text{a}}_{2}}\text{S}$ is a compound which have two atoms of sodium and one atom of sulphur. It is ionic in nature.
The reaction of sodium sulphide and sodium nitroprusside is used to detect presence of sulphur in organic compounds. Let us see the process and reactions involved-
Process: Take a small piece of a sodium metal and heat it gently in a fusion tube till it melts and shining globules are seen. A small amount of organic compound is added and the tube is heated till it becomes red hot. The red hot tube is plunged into distilled water contained in a china dish. Then, the contents are boiled, cooled and filtered. The filtrate is known as sodium extract. If an organic compound has sulphur and this sulphur reacts with sodium to form sodium sulphide. $\text{2Na}+\text{S}\to \text{N}{{\text{a}}_{2}}\text{S}$. This compound is present in extract.
Sodium nitroprusside test: Sodium nitroprusside solution is added to the sodium’s extract. The presence of sulphur is confirmed by the formation of sodium thio nitroprusside. The reactions involved is $\text{NaS}+\text{N}{{\text{a}}_{2}}\left[ \text{Fe}{{\left( \text{CN} \right)}_{5}}\text{NO} \right]\to \text{N}{{\text{a}}_{4}}\left[ \text{Fe}{{\left( \text{CN} \right)}_{5}}\text{NOS} \right]$. The compound formed is ‘purple’ in colour.
The colour developed when sodium sulphide is added to sodium nitroprusside is purple which is option ‘a’.
Additional Information:
Applications of sodium nitroprusside:
(1) Used in treatment of pulmonary hypertension and respiratory distress syndrome in new born babies.
(2) It is a medication to lower blood pressure.
(3) Used as analytical reagent for detection of amine, methyl ketones and thiols.
Note: The oxidation state of iron in sodium nitroprusside is +2. Iron is a diamagnetic low-spin centre with ${{\text{d}}^{6}}$ electronic configuration. It contains nitrosonium ligand which is positively charged and represented as $\text{N}{{\text{O}}^{+}}$. That’s why the oxidation state of iron is +2.
Complete answer:
Let us talk about sodium nitroprusside first to understand this compound.
It is a complex red-coloured compound that has an octahedral structure with iron(III) at the centre and surrounded by one linear nitric oxide ligand and five cyanide ligands. The structure of sodium nitroprusside is:

Sodium sulphide or $\text{N}{{\text{a}}_{2}}\text{S}$ is a compound which have two atoms of sodium and one atom of sulphur. It is ionic in nature.
The reaction of sodium sulphide and sodium nitroprusside is used to detect presence of sulphur in organic compounds. Let us see the process and reactions involved-
Process: Take a small piece of a sodium metal and heat it gently in a fusion tube till it melts and shining globules are seen. A small amount of organic compound is added and the tube is heated till it becomes red hot. The red hot tube is plunged into distilled water contained in a china dish. Then, the contents are boiled, cooled and filtered. The filtrate is known as sodium extract. If an organic compound has sulphur and this sulphur reacts with sodium to form sodium sulphide. $\text{2Na}+\text{S}\to \text{N}{{\text{a}}_{2}}\text{S}$. This compound is present in extract.
Sodium nitroprusside test: Sodium nitroprusside solution is added to the sodium’s extract. The presence of sulphur is confirmed by the formation of sodium thio nitroprusside. The reactions involved is $\text{NaS}+\text{N}{{\text{a}}_{2}}\left[ \text{Fe}{{\left( \text{CN} \right)}_{5}}\text{NO} \right]\to \text{N}{{\text{a}}_{4}}\left[ \text{Fe}{{\left( \text{CN} \right)}_{5}}\text{NOS} \right]$. The compound formed is ‘purple’ in colour.
The colour developed when sodium sulphide is added to sodium nitroprusside is purple which is option ‘a’.
Additional Information:
Applications of sodium nitroprusside:
(1) Used in treatment of pulmonary hypertension and respiratory distress syndrome in new born babies.
(2) It is a medication to lower blood pressure.
(3) Used as analytical reagent for detection of amine, methyl ketones and thiols.
Note: The oxidation state of iron in sodium nitroprusside is +2. Iron is a diamagnetic low-spin centre with ${{\text{d}}^{6}}$ electronic configuration. It contains nitrosonium ligand which is positively charged and represented as $\text{N}{{\text{O}}^{+}}$. That’s why the oxidation state of iron is +2.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




