
The colour of the phenolphthalein indicator in basic solution is:
(A) Yellow
(B) Green
(C) Pink
(D) Orange
Answer
588.9k+ views
Hint: Phenolphthalein is a weak acid and so it releases ${H^ + }$ ions in solution. The anion thus forms in coloured and indicates the endpoint. Adding a base to phenolphthalein causes the equilibrium to shift toward the acidic medium and release more ${H^ + }$ ions.
Complete step by step solution:
-Phenolphthalein is a synthetic indicator with a molecular formula of ${C_{20}}{H_{14}}{O_4}$. It belongs to the phthalein class of dyes and is most often used as an indicator in acid-base titrations. By nature, it has very low solubility in water and so for use in experiments, it is dissolved in alcohol.
Its structure is:
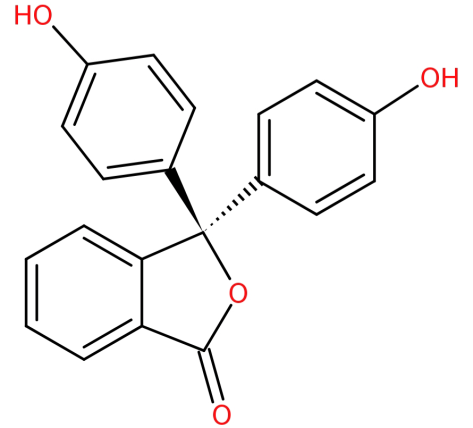
-It is formed by the condensation of phthalic anhydride with two equivalents of phenol under acidic conditions.
-It is a weak acid and so has the ability to lose ${H^ + }$ ions in solution. Before losing the ${H^ + }$ ion, the phenolphthalein molecule is colourless but after losing ${H^ + }$ ion it forms the phenolphthalein ion which is pink in colour.
According to Le-Chatelier's principle, if we add a base to phenolphthalein, the equilibrium will shift towards acidic medium leading to release of more ${H^ + }$ ions. Thus turning the solution from colourless to pink.
Hence we can say that phenolphthalein remains colourless in acidic and neutral solutions and turns pink in basic solutions. It remains colourless till pH of 8.5, while above that it turns pink to deep red in colour.
-In the liquid state it is colourless but when found in solid crystalline form phenolphthalein is yellowish-white to pale orange or fine white crystalline powder.
-Phenolphthalein is widely used as a pH indicator and a laboratory agent. It is also used in acid-base titrations as an indicator, medicinally as a laxative and in Kastle-Mayer test also. Along with methyl red, thymol blue and bromothymol blue, it can also act as a component of universal indicator.
So, the correct option will be: (C) Pink.
Note: It can adopt up to 4 different states in aqueous solutions due to the pH changes. When the conditions are strongly acidic, it exists in the protonated form ($H{\ln ^ + }$), which has orange colouration. When the conditions are between strongly acidic and slightly basic, it exists in lactone form (Hln) which is colourless. Under basic conditions it exists as doubly deprotonated phenolate form (${\ln ^{2 - }}$) which is colourless and under strongly basic conditions it forms $\ln {(OH)^{3 - }}$ form which is colourless.
Complete step by step solution:
-Phenolphthalein is a synthetic indicator with a molecular formula of ${C_{20}}{H_{14}}{O_4}$. It belongs to the phthalein class of dyes and is most often used as an indicator in acid-base titrations. By nature, it has very low solubility in water and so for use in experiments, it is dissolved in alcohol.
Its structure is:

-It is formed by the condensation of phthalic anhydride with two equivalents of phenol under acidic conditions.
-It is a weak acid and so has the ability to lose ${H^ + }$ ions in solution. Before losing the ${H^ + }$ ion, the phenolphthalein molecule is colourless but after losing ${H^ + }$ ion it forms the phenolphthalein ion which is pink in colour.
According to Le-Chatelier's principle, if we add a base to phenolphthalein, the equilibrium will shift towards acidic medium leading to release of more ${H^ + }$ ions. Thus turning the solution from colourless to pink.
Hence we can say that phenolphthalein remains colourless in acidic and neutral solutions and turns pink in basic solutions. It remains colourless till pH of 8.5, while above that it turns pink to deep red in colour.
-In the liquid state it is colourless but when found in solid crystalline form phenolphthalein is yellowish-white to pale orange or fine white crystalline powder.
-Phenolphthalein is widely used as a pH indicator and a laboratory agent. It is also used in acid-base titrations as an indicator, medicinally as a laxative and in Kastle-Mayer test also. Along with methyl red, thymol blue and bromothymol blue, it can also act as a component of universal indicator.
So, the correct option will be: (C) Pink.
Note: It can adopt up to 4 different states in aqueous solutions due to the pH changes. When the conditions are strongly acidic, it exists in the protonated form ($H{\ln ^ + }$), which has orange colouration. When the conditions are between strongly acidic and slightly basic, it exists in lactone form (Hln) which is colourless. Under basic conditions it exists as doubly deprotonated phenolate form (${\ln ^{2 - }}$) which is colourless and under strongly basic conditions it forms $\ln {(OH)^{3 - }}$ form which is colourless.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




