
The complex salt $CoCl_{ 4 }^{ 2- }$ has a tetrahedral structure. How many d-electrons are on the cobalt?
Answer
597.6k+ views
Hint: First just try to find the hybridization of the complex and then you can just calculate the electrons present. But keep in mind that if there is some charge left on the metal atom you have to satisfy that also. Now, you can easily solve this question.
Complete step by step answer:
First, we know that it is a tetrahedral complex so there is only one possibility of the hybridization of the molecule. That would be $sp^{ 3 }$.
In $sp^{ 3 }$ hybridization, we need one s and three p hybrid orbitals. These should be degenerate.
You should know that here $Cl^{ - }$ is a weak ligand, hence pairing is not possible.
Co has the atomic number 27, which means it has $3d^{ 7 }4s^{ 2 }$ electronic configuration.
In $CoCl_{ 4 }^{ 2- }$ complex, if we calculate the oxidation number of copper, it would be-
x + 4(-1) = -2
x = +2
So, here copper is in +2 oxidation state. That means it loses 2 electrons from 4s orbital.
After losing 2 electrons it is left with only 7 electrons and those are located in 3d orbitals.
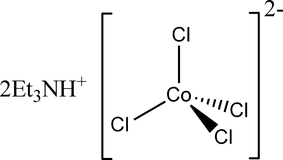
Therefore we can say that there are 7 d-electrons on the cobalt in complex salt $CoCl_{ 4 }^{ 2- }$.
Note: The geometry of $sp^{ 3 }$ Hybridization:
$sp^{ 3 }$ hybridized orbitals repel each other and they are directed to four corners of a regular tetrahedron. The angle between them is 109.5 degrees and the geometry of the molecule is tetrahedral (non-planar). This type of hybridization is also known as tetrahedral hybridization.
Complete step by step answer:
First, we know that it is a tetrahedral complex so there is only one possibility of the hybridization of the molecule. That would be $sp^{ 3 }$.
In $sp^{ 3 }$ hybridization, we need one s and three p hybrid orbitals. These should be degenerate.
You should know that here $Cl^{ - }$ is a weak ligand, hence pairing is not possible.
Co has the atomic number 27, which means it has $3d^{ 7 }4s^{ 2 }$ electronic configuration.
In $CoCl_{ 4 }^{ 2- }$ complex, if we calculate the oxidation number of copper, it would be-
x + 4(-1) = -2
x = +2
So, here copper is in +2 oxidation state. That means it loses 2 electrons from 4s orbital.
After losing 2 electrons it is left with only 7 electrons and those are located in 3d orbitals.

Therefore we can say that there are 7 d-electrons on the cobalt in complex salt $CoCl_{ 4 }^{ 2- }$.
Note: The geometry of $sp^{ 3 }$ Hybridization:
$sp^{ 3 }$ hybridized orbitals repel each other and they are directed to four corners of a regular tetrahedron. The angle between them is 109.5 degrees and the geometry of the molecule is tetrahedral (non-planar). This type of hybridization is also known as tetrahedral hybridization.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




