
The compound gallium arsenide is a commonly used semiconductor, having an energy gap E of 1.43eV. Its crystal structure is like silicon, except that half the silicon atoms are replaced by gallium atoms and half by arsenic atoms. Draw a flattened-out sketch of the gallium arsenide lattice, following the pattern of Fig. What is the net charge of the (a) gallium and (b) arsenic ion core? (c) How many electrons per bond are there?
(Hint: Consult the periodic table in Appendix G)

Answer
460.5k+ views
Hint: Gallium and Arsenic are located in the third and the fifth column of the periodic table. Gallium arsenide is a direct-gap semiconductor. However, gallium arsenide devices are less sensitive to heat since they have a large bandgap.
Complete answer:
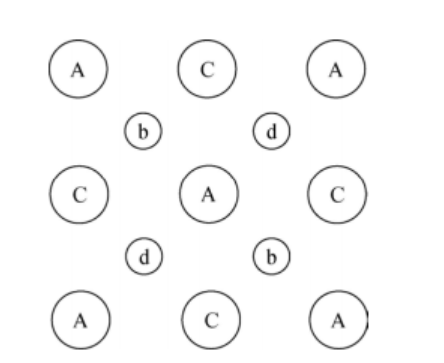
There is a covalent bond present between the arsenic atom and the gallium atom. The figure shows that each gallium atom is connected to four arsenic atoms, and each arsenic atom is connected to four gallium atoms. In the given figure, the depth of the lattice structure cannot be estimated because the figure is a flattened representation of silicon.
As per Miller indices, the view shown in the figure is of 1, 0, 0 type, and we will use alphabets to specify the depth of the atoms.
Let's indicate the atoms with the alphabets such as:
The capital letters in the figure are used to determine the Gallium atoms, and the small letters are used to determine the arsenic atoms.
Observe the arsenic atom (the letter b) in the upper left corner, which has covalent bonds with the two A's and two C's nearby. Notice the arsenic atom (with the letter d) near the upper right; it has covalent bonds with the two C's nearby and the two E's (behind the A's nearby).
Let us answer the questions asked one by one.
a).What is the net charge of the gallium?
Arsenic and gallium have completely filled 3p, 3d and 4s subshells. However, their 4p subshell is partially filled. A neutral gallium atom that is isolated has one electron in a 4p subshell, and the arsenic atom has five electrons in the 4p subshell. To gain a stable net charge, it needs 8 electrons because four bonds are connected to each atom in the lattice. So, electrons will be shared from the 4p subshell, and the 4s subshell will also give its electrons. The outer orbital loses 1 electron, and the 4s subshell loses 2 electrons to gain a net charge of +3e.
(b).What is the net charge of arsenic?
The arsenic loses three electrons from the 4p subshell and two electrons from the 4s subshell. So the net charge on the arsenic ion is +5e.
(c) How many electrons per bond are there?
In the covalent bond formed in the figure, two electrons are shared per bond.
The flattened-out sketch of the gallium arsenide lattice will be the same as silicon:
Note:
Unlike many other semiconductors, GaAs has a straight bandgap, which can emit light efficiently. Its employment in optical windows and space electronics in high-power applications is enabled because it is a direct bandgap substance that is immune to radiation damage.
Complete answer:
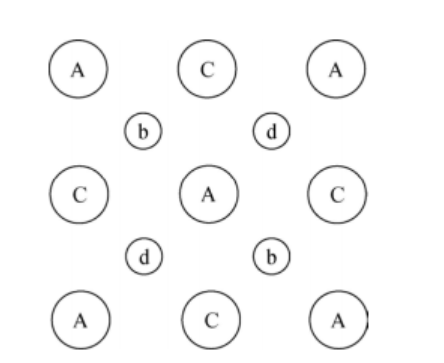
There is a covalent bond present between the arsenic atom and the gallium atom. The figure shows that each gallium atom is connected to four arsenic atoms, and each arsenic atom is connected to four gallium atoms. In the given figure, the depth of the lattice structure cannot be estimated because the figure is a flattened representation of silicon.
As per Miller indices, the view shown in the figure is of 1, 0, 0 type, and we will use alphabets to specify the depth of the atoms.
Let's indicate the atoms with the alphabets such as:
| Letter | Specification |
| A | closest atoms near to the observer |
| B | the next deep layer |
| C | further embedded into the page |
| D | the last layer seen |
| E | (not visible) for the atoms that are at the deepest layer (they are behind the A's) |
The capital letters in the figure are used to determine the Gallium atoms, and the small letters are used to determine the arsenic atoms.
Observe the arsenic atom (the letter b) in the upper left corner, which has covalent bonds with the two A's and two C's nearby. Notice the arsenic atom (with the letter d) near the upper right; it has covalent bonds with the two C's nearby and the two E's (behind the A's nearby).
Let us answer the questions asked one by one.
a).What is the net charge of the gallium?
Arsenic and gallium have completely filled 3p, 3d and 4s subshells. However, their 4p subshell is partially filled. A neutral gallium atom that is isolated has one electron in a 4p subshell, and the arsenic atom has five electrons in the 4p subshell. To gain a stable net charge, it needs 8 electrons because four bonds are connected to each atom in the lattice. So, electrons will be shared from the 4p subshell, and the 4s subshell will also give its electrons. The outer orbital loses 1 electron, and the 4s subshell loses 2 electrons to gain a net charge of +3e.
(b).What is the net charge of arsenic?
The arsenic loses three electrons from the 4p subshell and two electrons from the 4s subshell. So the net charge on the arsenic ion is +5e.
(c) How many electrons per bond are there?
In the covalent bond formed in the figure, two electrons are shared per bond.
The flattened-out sketch of the gallium arsenide lattice will be the same as silicon:

Note:
Unlike many other semiconductors, GaAs has a straight bandgap, which can emit light efficiently. Its employment in optical windows and space electronics in high-power applications is enabled because it is a direct bandgap substance that is immune to radiation damage.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




