
The compound which reacts fastest with Lucas reagent at room temperature is:
1. butan-1-ol
2. butan-2-ol
3. 2-methylpropan-1-ol
4. 2.methylpropane-2-ol
Answer
585.3k+ views
Hint: Here we should focus on the stability of carbocation, since when the above compounds will react with Lucas reagent, they will form a carbocation intermediate, and our aim is to select the most stable form of carbocation.
Complete step by step solution: Let's first decide on the stability of carbocation:
- Carbocation is classified as primary, secondary, or tertiary depending on one, two or three carbons directly attached to the positively charged carbon in the chain.
- So, the order of stability: primary carbocation < secondary carbocation < tertiary carbocation
Now let's briefly know the function of Lucas reagent:
- Alcohols at room temperature react with an equimolar mixture of concentrated hydrochloric acid and anhydrous zinc chloride to form alkyl halides, which is known as Lucas reagent.
- The equation is: $ROH+\underset{Conc.}{\mathop{HCl}}\,\xrightarrow{\text{Anhydrous ZnC}{{\text{l}}_{\text{2}}}}R-Cl+{{H}_{2}}O$
- On reacting with this reagent, an alkyl halide is formed.
- The rates of reaction by this reagent follow the following order: primary alcohol < secondary alcohol < tertiary alcohol
- As this stability order also depends on the degree of carbocation formed.
So, now let's look into the above question stated:
- The first option here is butan-1-ol, which is a primary alcohol, and forms a 1 degree carbocation, thus is not much stable.
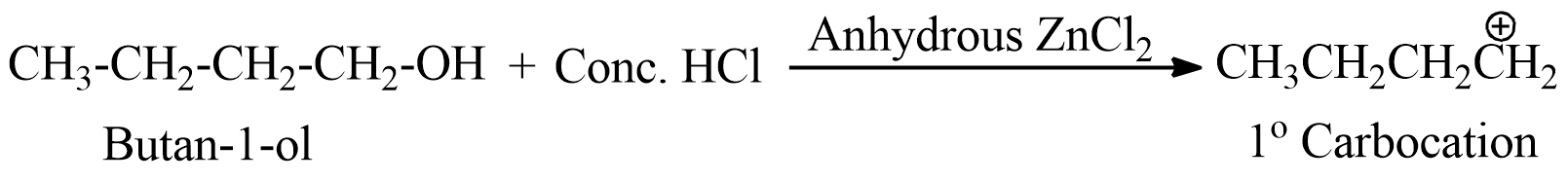
- The second option has butan-2-ol, which forms secondary carbocation, and is more stable than primary one.
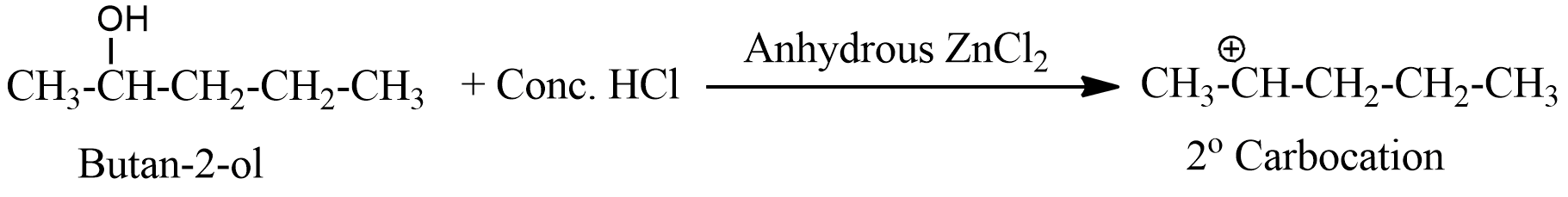
- The third option has 2-methylpropan-1-ol, this is also a primary alcohol, and forms a primary carbocation, and also does not react faster with Lucas reagent.
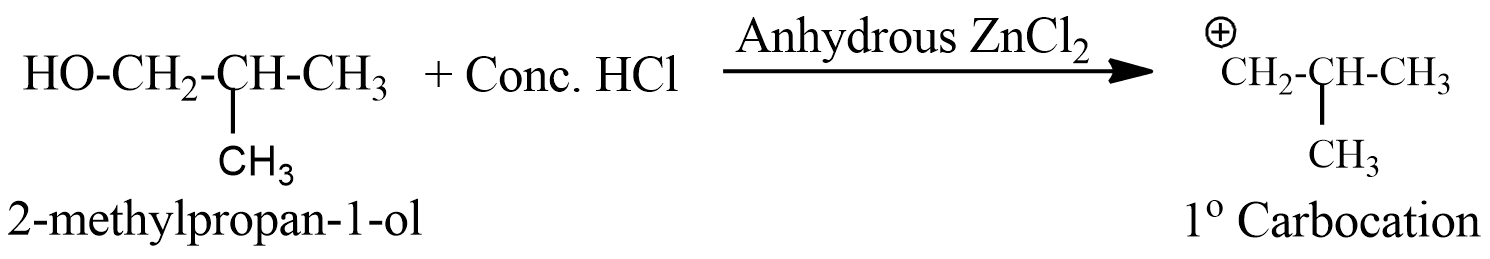
- But the fourth option has 2-methylpropan-2-ol, which forms a tertiary carbocation when reacts with Lucas reagent and thus is the most stable.
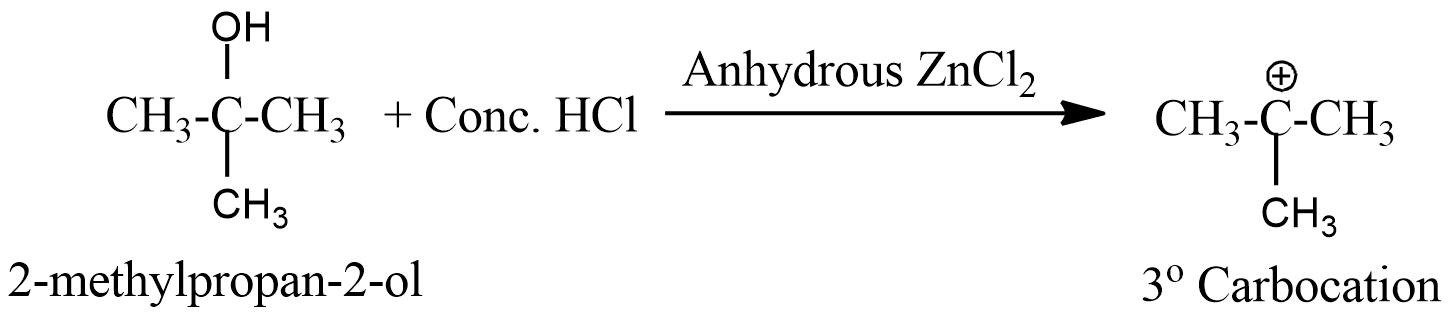
- So option D reacts fastest with the Lucas reagent and is the correct answer.
Additional Information: Now when the Lucas reagent is made to react with an unknown alcohol, the solution becomes cloudy before it separates as a distinct layer, and we can observe the following:
- If the turbidity (white) appears immediately, it's a tertiary alcohol.
- If turbidity appears within 5 minutes, it's a secondary alcohol.
- If the solution remains clear, and shows no turbidity, it's a primary alcohol.
Note: During the reaction of an alcohol with Lucas reagent, an alkyl halide is formed. So we need to check the type of carbocation intermediate formed during the reaction, when the hydroxyl group escapes out in the form of one water molecule and that will decide which alcohol will react fastest to form carbocation.
Complete step by step solution: Let's first decide on the stability of carbocation:
- Carbocation is classified as primary, secondary, or tertiary depending on one, two or three carbons directly attached to the positively charged carbon in the chain.
- So, the order of stability: primary carbocation < secondary carbocation < tertiary carbocation
Now let's briefly know the function of Lucas reagent:
- Alcohols at room temperature react with an equimolar mixture of concentrated hydrochloric acid and anhydrous zinc chloride to form alkyl halides, which is known as Lucas reagent.
- The equation is: $ROH+\underset{Conc.}{\mathop{HCl}}\,\xrightarrow{\text{Anhydrous ZnC}{{\text{l}}_{\text{2}}}}R-Cl+{{H}_{2}}O$
- On reacting with this reagent, an alkyl halide is formed.
- The rates of reaction by this reagent follow the following order: primary alcohol < secondary alcohol < tertiary alcohol
- As this stability order also depends on the degree of carbocation formed.
So, now let's look into the above question stated:
- The first option here is butan-1-ol, which is a primary alcohol, and forms a 1 degree carbocation, thus is not much stable.
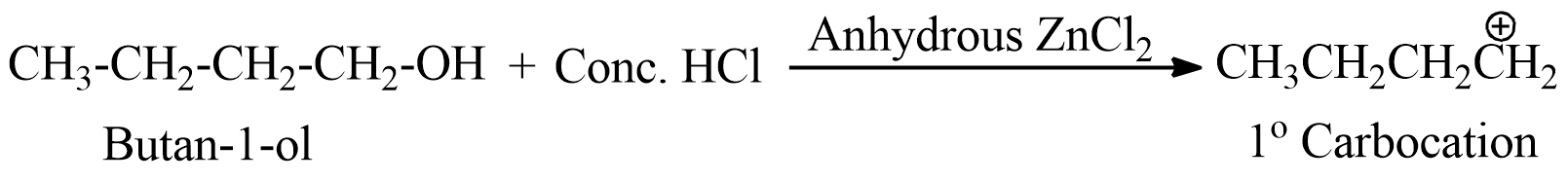
- The second option has butan-2-ol, which forms secondary carbocation, and is more stable than primary one.

- The third option has 2-methylpropan-1-ol, this is also a primary alcohol, and forms a primary carbocation, and also does not react faster with Lucas reagent.
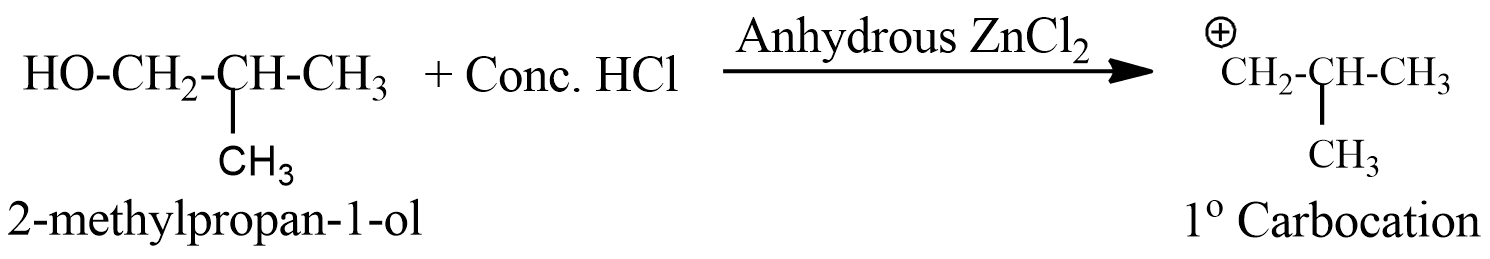
- But the fourth option has 2-methylpropan-2-ol, which forms a tertiary carbocation when reacts with Lucas reagent and thus is the most stable.

- So option D reacts fastest with the Lucas reagent and is the correct answer.
Additional Information: Now when the Lucas reagent is made to react with an unknown alcohol, the solution becomes cloudy before it separates as a distinct layer, and we can observe the following:
- If the turbidity (white) appears immediately, it's a tertiary alcohol.
- If turbidity appears within 5 minutes, it's a secondary alcohol.
- If the solution remains clear, and shows no turbidity, it's a primary alcohol.
Note: During the reaction of an alcohol with Lucas reagent, an alkyl halide is formed. So we need to check the type of carbocation intermediate formed during the reaction, when the hydroxyl group escapes out in the form of one water molecule and that will decide which alcohol will react fastest to form carbocation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




