
The compound with the above configuration is called__________.
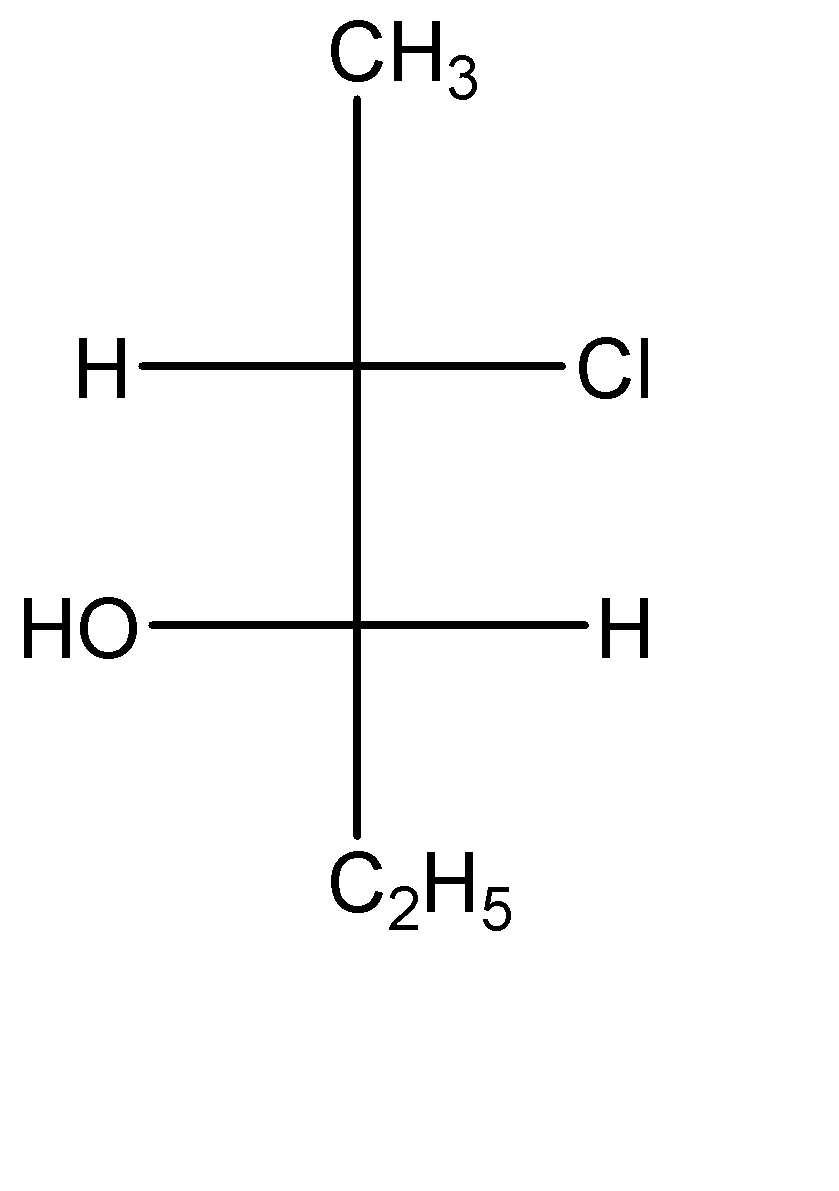
(A) (2S, 3S)-2-chloro-3-hydroxypentane
(B) (2S, 3R)-2-chloro-3-hydroxypentane
(C) (2R, 3R)-2-chloro-3-hydroxypentane
(D) (2R, 3S)-2-chloro-3-hydroxypentane

Answer
522.9k+ views
Hint: As we know that R and S notation use the CIP priority rules for assigning the absolute configuration around a stereocenter and this concept was proposed by Rosanoff. Since the last name of the compounds in the given options is the same, we just need to focus on finding the absolute configuration of stereocenter or chiral carbons (carbon which is attached to 4 different substituents).
Complete answer:
Let us solve this question as follows:-
- Firstly assign priorities to groups attached around the stereocentre or chiral carbon from 1 to 4 where 1 being the highest and 4 being the lowest priority.
- Now follow the direction from 1 to 2 and 2 to 3 in a circular way. If it is in clockwise manner, then the configuration is R (rectus) and if it is in anticlockwise manner, then the configuration is S (sinister).
-Rules for priority order are as follows:-
(I) Priority is given on the basis of atomic number, higher the atomic number, higher the priority.
(II) When two or more substituents have the same first atom, then move to the next atom attached to it and compare their atomic numbers.
(III) If a lower priority group is in the horizontal line of Fischer projection, then the configuration is opposite to the circular way.
-Now let us check this compound:-
In the second carbon (which is a stereocenter), the circular way tells clockwise configuration which means ‘R’, but since the lowest priority group is in horizontal line, so the absolute configuration of this carbon is ‘S’.
In the third carbon (which is a stereocenter) also, the circular way tells clockwise configuration which means ‘R’, but since the lowest priority group is in horizontal line, so the absolute configuration of this carbon is ‘S’ as well.
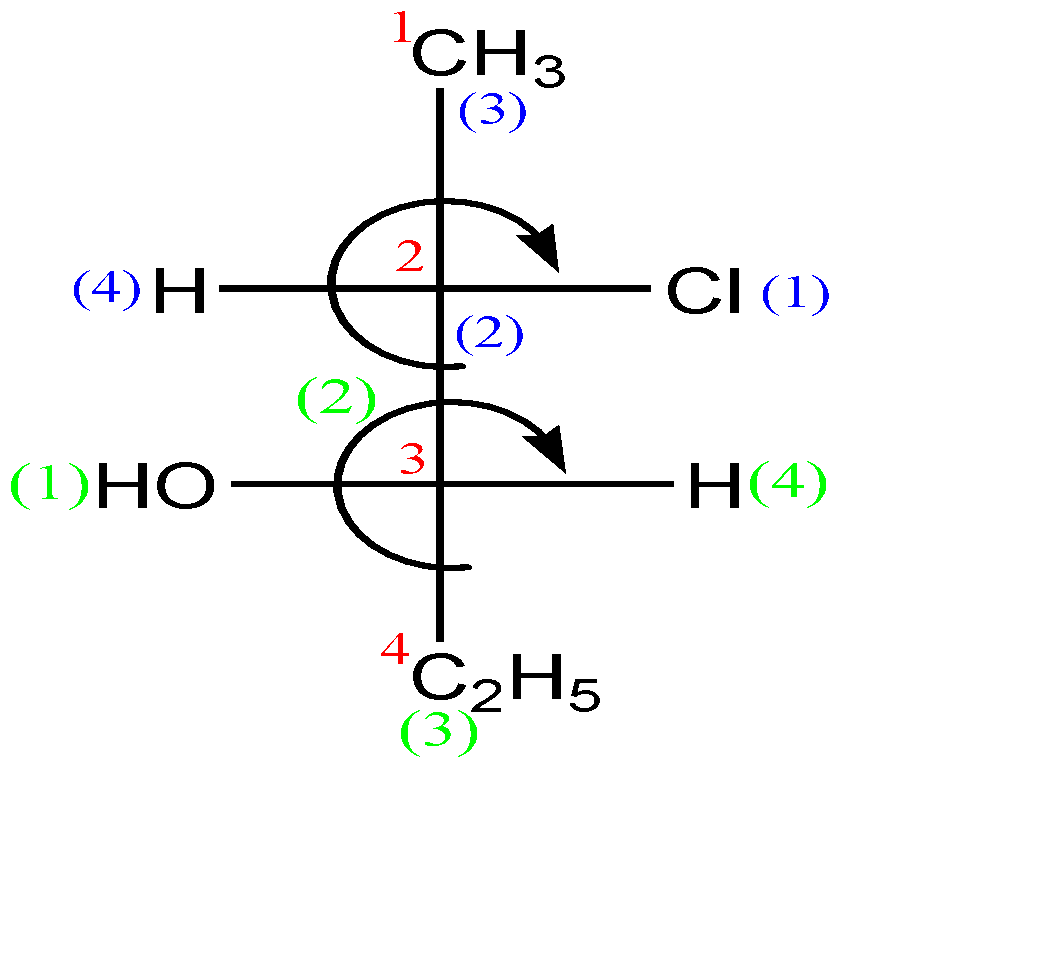
Hence the name of the compound along with configuration is: (A) (2S, 3S)-2-chloro-3-hydroxypentane.
Note:
-While giving priorities, remember that if two or more groups are attached to isotopes of the same element (have same atomic number), so assign them according to their atomic masses.
-Also to solve such types of questions, understand the concept of conformers and configurations.
Complete answer:
Let us solve this question as follows:-
- Firstly assign priorities to groups attached around the stereocentre or chiral carbon from 1 to 4 where 1 being the highest and 4 being the lowest priority.
- Now follow the direction from 1 to 2 and 2 to 3 in a circular way. If it is in clockwise manner, then the configuration is R (rectus) and if it is in anticlockwise manner, then the configuration is S (sinister).
-Rules for priority order are as follows:-
(I) Priority is given on the basis of atomic number, higher the atomic number, higher the priority.
(II) When two or more substituents have the same first atom, then move to the next atom attached to it and compare their atomic numbers.
(III) If a lower priority group is in the horizontal line of Fischer projection, then the configuration is opposite to the circular way.
-Now let us check this compound:-
In the second carbon (which is a stereocenter), the circular way tells clockwise configuration which means ‘R’, but since the lowest priority group is in horizontal line, so the absolute configuration of this carbon is ‘S’.
In the third carbon (which is a stereocenter) also, the circular way tells clockwise configuration which means ‘R’, but since the lowest priority group is in horizontal line, so the absolute configuration of this carbon is ‘S’ as well.

Hence the name of the compound along with configuration is: (A) (2S, 3S)-2-chloro-3-hydroxypentane.
Note:
-While giving priorities, remember that if two or more groups are attached to isotopes of the same element (have same atomic number), so assign them according to their atomic masses.
-Also to solve such types of questions, understand the concept of conformers and configurations.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




