
The conformations of n-butane, commonly known as eclipsed, gauche and anti conformations can be interconverted by :
a.) Rotation around $C - H$ bond of a methyl group
b.) Rotation around $C - H$ bond of a methylene group
c.) Rotation around $C1 - C2$ linkage
d.) Rotation around $C2 - C3$ linkage
Answer
594k+ views
Hint : The conformations of butane can be interconverted only by rotation around the central carbon atoms in butane.
Complete answer :
Butane is a form of carbon molecule that exists in three forms when we see molecules in Newman projection or sawhorse projection.
Conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about single bonds. These conformations change only by single carbon carbon rotation. In butane, four such conformations are possible which are fully eclipsed, eclipsed, gauche and anti.
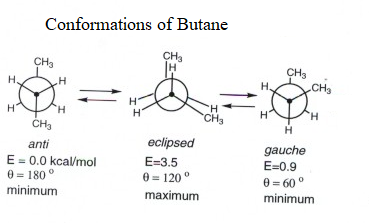
The three conformations of butane given in question i.e. eclipsed, anti and gauche can be interconverted by rotation around $C2 - C3$ linkage.
Thus, option d.) is the correct answer.
The rotations around C-H in methyl will not show any difference. SO, option a.) and b.) can not be the answer.
Additional information :
From the diagram we can also conclude that anti conformation is most stable because in anti conformation, the two methyl groups on both carbon atoms are placed at dihedral angle of 180. Thus, the steric hindrance is minimum in this position.
Note : If we make Newman projection using carbon 1 and 2 then the front carbon will contain H atoms only and they do not impose steric hindrance and thus, such conformations are not always possible.
Complete answer :
Butane is a form of carbon molecule that exists in three forms when we see molecules in Newman projection or sawhorse projection.
Conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about single bonds. These conformations change only by single carbon carbon rotation. In butane, four such conformations are possible which are fully eclipsed, eclipsed, gauche and anti.

The three conformations of butane given in question i.e. eclipsed, anti and gauche can be interconverted by rotation around $C2 - C3$ linkage.
Thus, option d.) is the correct answer.
The rotations around C-H in methyl will not show any difference. SO, option a.) and b.) can not be the answer.
Additional information :
From the diagram we can also conclude that anti conformation is most stable because in anti conformation, the two methyl groups on both carbon atoms are placed at dihedral angle of 180. Thus, the steric hindrance is minimum in this position.
Note : If we make Newman projection using carbon 1 and 2 then the front carbon will contain H atoms only and they do not impose steric hindrance and thus, such conformations are not always possible.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




