
The coordination number and the oxidation state of the element E in the complex $[E{(en)_2}({C_2}{O_4})]N{O_2}$ (where (en) is ethylene diamine) are respectively:
A) 4 and 3
B) 6 and 3
C) 6 and 2
D) 4 and 2
Answer
576.3k+ views
Hint:. The total number of coordinate bonds formed by the ligands in the complex $[E{(en)_2}({C_2}{O_4})]N{O_2}$ with the metal ion (here, element E) will be the coordination number of E. Oxidation state of element E in the given complex can be calculated by adding the charges of all the ligands and the counter ion present in the coordination entity.
Complete step by step answer:
We are given a complex, $[E{(en)_2}({C_2}{O_4})]N{O_2}$, where (en) is ethylene diamine. E is the metal ion in the complex, en and ${C_2}{O_4}^{2 - }$ (oxalate ion) are ligands joined to metal ion E with the coordinate covalent bonds. $N{O_2}$ is the counter ion.
- Coordination number: The total number of coordinate bonds formed by the ligands in the complex with the metal ion is called coordination number or it can also be defined as the number of ligand donor atoms to which the metal ion is directly bonded.
Now, in the given complex, ligands are en (ethylene diamine) and ${C_2}{O_4}^{2 - }$ (oxalate ion) and their structure is as follows:
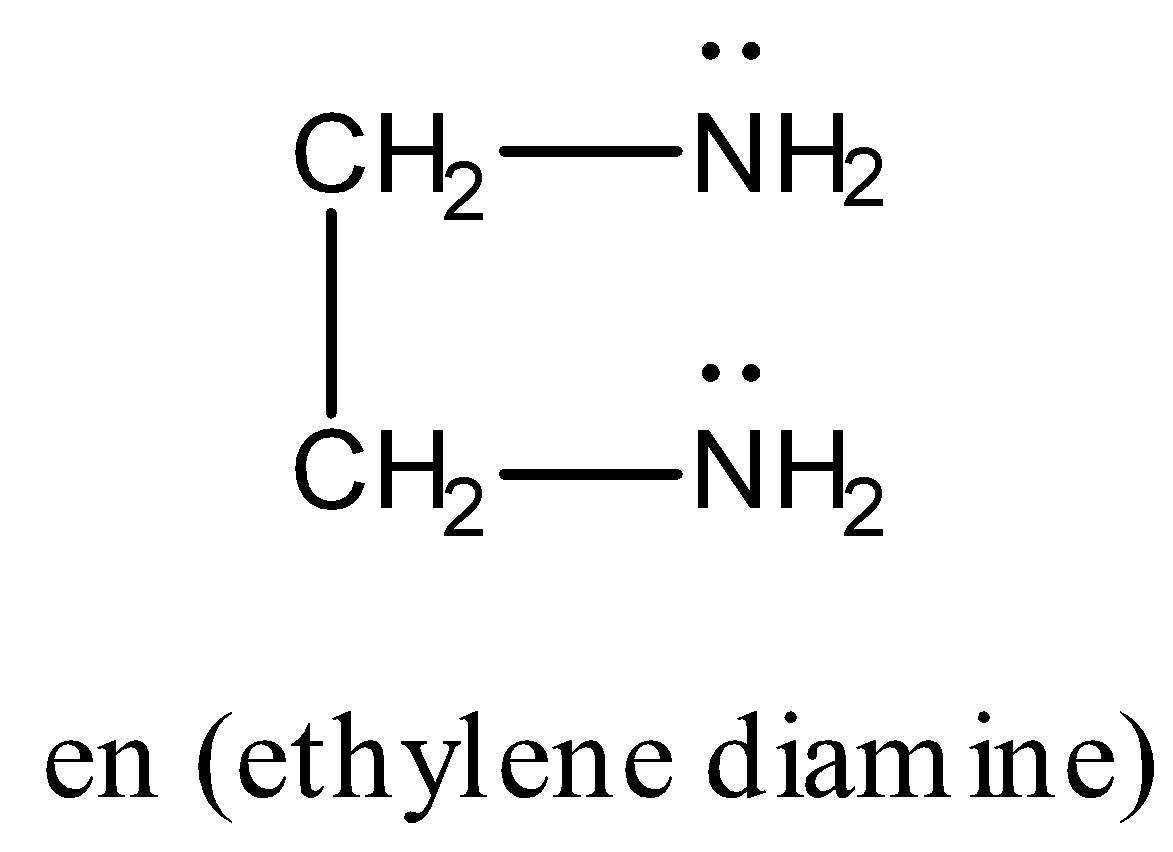
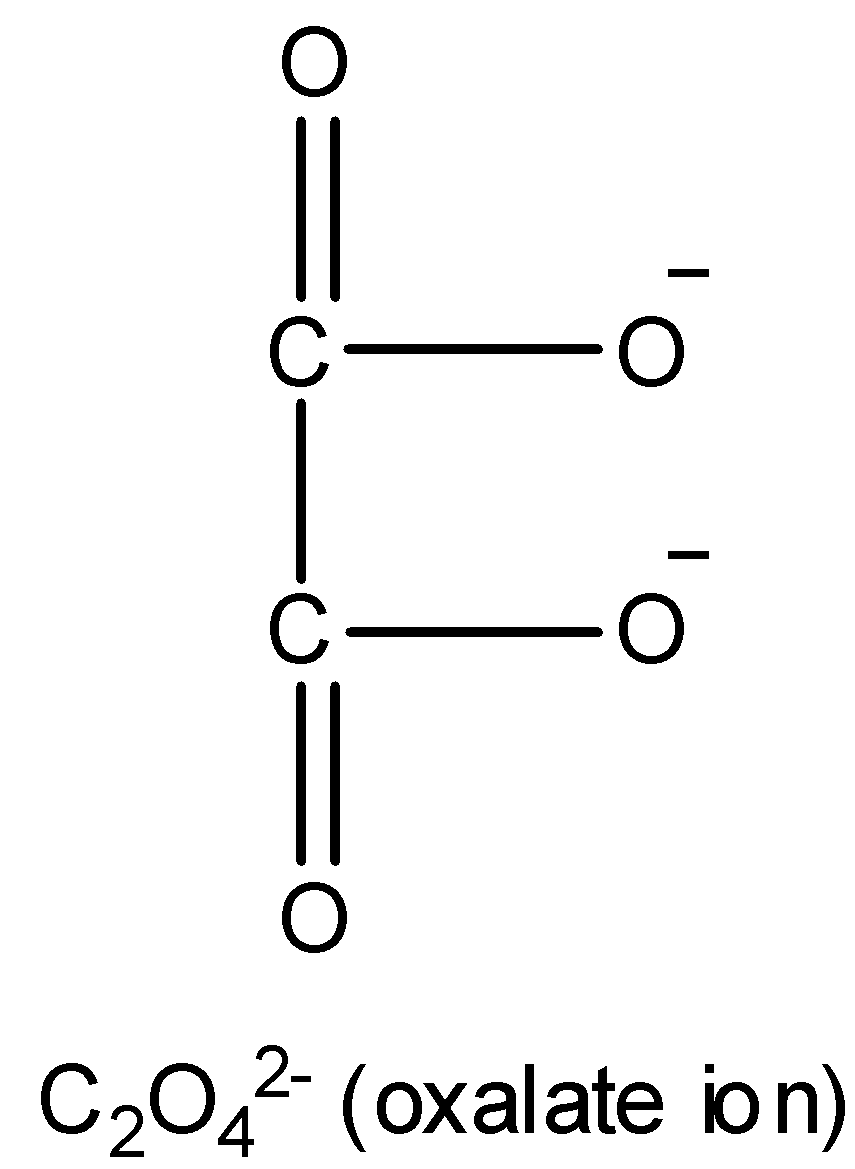
Both ligands en and ${C_2}{O_4}^{2 - }$ are bidentate ligands because they both have two donor atoms that can bind to the metal E. Donor atoms in en are two nitrogen atoms while donor atoms in ${C_2}{O_4}^{2 - }$ are two oxygen atoms.
Thus in complex $[E{(en)_2}({C_2}{O_4})]N{O_2}$, two en ligands will bind to the metal E with four coordinate bonds and one ${C_2}{O_4}^{2 - }$ ligand will bind to the metal E with two coordinate bonds. Therefore, the total number of coordinate bonds formed by the ligands in the complex with the metal ion are $4 + 2 = 6$ . Hence, 6 is called coordination number of the element E in the complex.
- We know that oxidation states of an element can be calculated by adding the charges of all the ligands and the counter ions (i.e., ions present outside the central atom).
Ligand en is a neutral ligand, therefore charge on en is 0. Charge on ligand ${C_2}{O_4}^{2 - }$ is -2 and on the counter ion i.e. $N{O_2}$ is -1. Therefore, the oxidation state of element E (let $x$) in the complex, $[E{(en)_2}({C_2}{O_4})]N{O_2}$ is:
$x + (2 \times 0) + ( - 1) + ( - 1) = 0$
$x - 2 - 1 = 0$
$x = + 3$
Thus, the oxidation state of E in the complex is +3.
So, the correct answer is “Option B”.
Note: It should be known that when a ligand binds to the central metal ion through two donor atoms as in en and ${C_2}{O_4}^{2 - }$, the ligand is said to be bidentate or tridentate ligand. The oxidation number is represented by a roman numeral in the naming of the coordination complex. For example, the oxidation number of E in $[E{(en)_2}({C_2}{O_4})]N{O_2}$ is +3 and it is written as E(III).
Complete step by step answer:
We are given a complex, $[E{(en)_2}({C_2}{O_4})]N{O_2}$, where (en) is ethylene diamine. E is the metal ion in the complex, en and ${C_2}{O_4}^{2 - }$ (oxalate ion) are ligands joined to metal ion E with the coordinate covalent bonds. $N{O_2}$ is the counter ion.
- Coordination number: The total number of coordinate bonds formed by the ligands in the complex with the metal ion is called coordination number or it can also be defined as the number of ligand donor atoms to which the metal ion is directly bonded.
Now, in the given complex, ligands are en (ethylene diamine) and ${C_2}{O_4}^{2 - }$ (oxalate ion) and their structure is as follows:


Both ligands en and ${C_2}{O_4}^{2 - }$ are bidentate ligands because they both have two donor atoms that can bind to the metal E. Donor atoms in en are two nitrogen atoms while donor atoms in ${C_2}{O_4}^{2 - }$ are two oxygen atoms.
Thus in complex $[E{(en)_2}({C_2}{O_4})]N{O_2}$, two en ligands will bind to the metal E with four coordinate bonds and one ${C_2}{O_4}^{2 - }$ ligand will bind to the metal E with two coordinate bonds. Therefore, the total number of coordinate bonds formed by the ligands in the complex with the metal ion are $4 + 2 = 6$ . Hence, 6 is called coordination number of the element E in the complex.
- We know that oxidation states of an element can be calculated by adding the charges of all the ligands and the counter ions (i.e., ions present outside the central atom).
Ligand en is a neutral ligand, therefore charge on en is 0. Charge on ligand ${C_2}{O_4}^{2 - }$ is -2 and on the counter ion i.e. $N{O_2}$ is -1. Therefore, the oxidation state of element E (let $x$) in the complex, $[E{(en)_2}({C_2}{O_4})]N{O_2}$ is:
$x + (2 \times 0) + ( - 1) + ( - 1) = 0$
$x - 2 - 1 = 0$
$x = + 3$
Thus, the oxidation state of E in the complex is +3.
So, the correct answer is “Option B”.
Note: It should be known that when a ligand binds to the central metal ion through two donor atoms as in en and ${C_2}{O_4}^{2 - }$, the ligand is said to be bidentate or tridentate ligand. The oxidation number is represented by a roman numeral in the naming of the coordination complex. For example, the oxidation number of E in $[E{(en)_2}({C_2}{O_4})]N{O_2}$ is +3 and it is written as E(III).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




