
The correct basicity order of the following compound is:
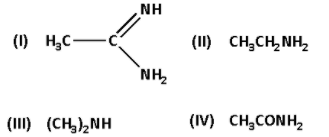
A) I > III > II > IV
B) III > I > II > IV
C) II > I > III > IV
D) I > II > III > IV

Answer
566.7k+ views
Hint:More the number of the lone pair of electrons are available higher is the basicity. The basicity is directly proportional to the number of electron donating groups and is inversely proportional to the number of electron withdrawing groups.
Complete step-by-step answer:
In compound (I), there is a lone pair of electron on both the groups ${\text{N}}{{\text{H}}_{\text{2}}}$ and ${\text{NH}}$. The lone pair of electrons on ${\text{N}}{{\text{H}}_{\text{2}}}$ group is in conjugation and is not available. But the lone pair of electrons on ${\text{NH}}$ group is not in conjugation and is available. Also, the two carbon atoms are electron donating. Thus, there are two electron donating groups.
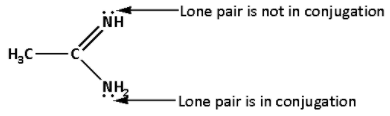
Thus, in compound (I), there is one lone pair available, two electron donating groups and also conjugation is available.
In compound (II), there is a lone pair on the ${\text{N}}{{\text{H}}_{\text{2}}}$ group. The lone pair of electrons on ${\text{N}}{{\text{H}}_{\text{2}}}$ group is not in conjugation and is available. Also, the two carbon atoms are electron donating. Thus, there are two electron donating groups from one side of the functional group.
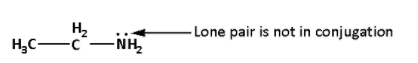
Thus, in compound (II), there is one lone pair available, two electron donating groups from one side of the functional group but there is no conjugation.Thus, compound (I) is more basic than compound (II).
In compound (III), there is a lone pair on the ${\text{NH}}$ group. The lone pair of electrons on the ${\text{NH}}$ group is not in conjugation and is available. Also, the two carbon atoms are electron donating. Thus, there are two electron donating groups from both sides of the functional group.
Thus, in compound (III), there is one lone pair available, two electron donating groups from both sides of the functional group.

Thus, compound (III) is more basic than compound (II).
In compound (IV), there is a lone pair of electrons on the ${\text{N}}{{\text{H}}_{\text{2}}}$ group. The lone pair of electrons on ${\text{N}}{{\text{H}}_{\text{2}}}$ group is in conjugation and is not available. The group $\left( {{\text{C}} = {\text{O}}} \right)$ is an electron withdrawing group.
Thus, in compound (IV), there is no lone pair available and also, there is an electron withdrawing group which decreases the basicity.
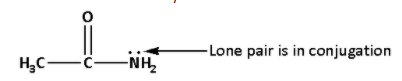
Thus, compound (IV) is least basic. Thus, the correct basicity order is as follows:
I > III > II > IV
Thus, the correct answer is (A) I > III > II > IV.
Note: We should remember that
i) As the number of the electron donating groups increases the basicity of the compound increases.
ii) As the number of electron withdrawing groups increases the basicity of the compound decreases.
Complete step-by-step answer:
In compound (I), there is a lone pair of electron on both the groups ${\text{N}}{{\text{H}}_{\text{2}}}$ and ${\text{NH}}$. The lone pair of electrons on ${\text{N}}{{\text{H}}_{\text{2}}}$ group is in conjugation and is not available. But the lone pair of electrons on ${\text{NH}}$ group is not in conjugation and is available. Also, the two carbon atoms are electron donating. Thus, there are two electron donating groups.

Thus, in compound (I), there is one lone pair available, two electron donating groups and also conjugation is available.
In compound (II), there is a lone pair on the ${\text{N}}{{\text{H}}_{\text{2}}}$ group. The lone pair of electrons on ${\text{N}}{{\text{H}}_{\text{2}}}$ group is not in conjugation and is available. Also, the two carbon atoms are electron donating. Thus, there are two electron donating groups from one side of the functional group.
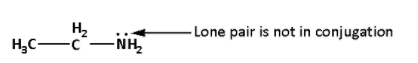
Thus, in compound (II), there is one lone pair available, two electron donating groups from one side of the functional group but there is no conjugation.Thus, compound (I) is more basic than compound (II).
In compound (III), there is a lone pair on the ${\text{NH}}$ group. The lone pair of electrons on the ${\text{NH}}$ group is not in conjugation and is available. Also, the two carbon atoms are electron donating. Thus, there are two electron donating groups from both sides of the functional group.
Thus, in compound (III), there is one lone pair available, two electron donating groups from both sides of the functional group.

Thus, compound (III) is more basic than compound (II).
In compound (IV), there is a lone pair of electrons on the ${\text{N}}{{\text{H}}_{\text{2}}}$ group. The lone pair of electrons on ${\text{N}}{{\text{H}}_{\text{2}}}$ group is in conjugation and is not available. The group $\left( {{\text{C}} = {\text{O}}} \right)$ is an electron withdrawing group.
Thus, in compound (IV), there is no lone pair available and also, there is an electron withdrawing group which decreases the basicity.

Thus, compound (IV) is least basic. Thus, the correct basicity order is as follows:
I > III > II > IV
Thus, the correct answer is (A) I > III > II > IV.
Note: We should remember that
i) As the number of the electron donating groups increases the basicity of the compound increases.
ii) As the number of electron withdrawing groups increases the basicity of the compound decreases.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




