
The correct energy level diagram for ${{\left[ Co{{\left( CN \right)}_{6}} \right]}^{3-}}$,
A)
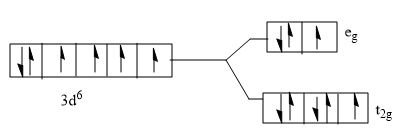
B)
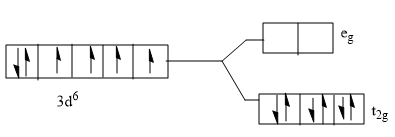
C)
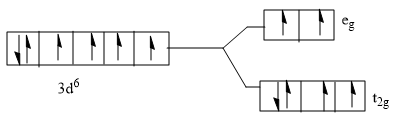
D)
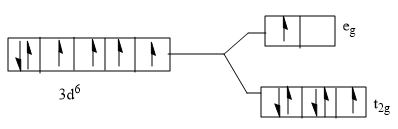




Answer
565.2k+ views
Hint: Cyanide ion is a strong ligand and strong ligands splits up the d-orbitals more.
${{t}_{2}}g$ should be filled with at least one electron in each orbitals to fill up in the higher ${{e}_{g}}$ orbital.
Complete step by step answer:
In the question it is given, a complex of Co. Co is the central metal atom and the cyanide ions are the ligands that are surrounding the Co atom, there are six cyanide ions and hence we can say that it is an octahedral complex.
- According to the spectrochemical series the cyanide ion is one of the strongest ligands carrying a charge of -1.
- And now we have to know that the Co is in which oxidation state, either we can find through the calculation method but here it is already given in the options that it is a $3{{d}^{6}}$ system.
- If it wasn’t given, then we calculate the oxidation State and find the ${{d}^{n}}$ system to which the complex belongs to.
For that in the complex give the oxidation number of Co as x.
${{\left[ Co{{\left( CN \right)}_{6}} \right]}^{3-}}$-oxidation state- $x+\left( -1 \right)(6)=-3$, x = 3
So here Co is in +3 oxidation state.
The electronic configuration of Co is -$\left[ Ar \right]3{{d}^{7}}4{{s}^{2}}$
In +3 state, it will have a configuration as $\left[ Ar \right]3{{d}^{6}}$
So the system is ${{d}^{6}}$ system.
- Now we know that in d orbitals are divided into 2 levels, ${{t}_{2}}g$ having a set of 3 orbitals ${{d}_{xy}},{{d}_{yz}},{{d}_{xz}}$ and ${{e}_{g}}$ with two orbitals ${{d}_{{{x}^{2}}-{{y}^{2}}}},{{d}_{{{z}^{2}}}}$
- As this complex is an octahedral complex, ${{t}_{2}}g$ will be the orbital with lower energy level and ${{e}_{g}}$ is the higher energy level
- As $C{{N}^{-}}$ is a strong ligand it splits up, ${{t}_{2}}g$ and ${{e}_{g}}$ in a greater energy, splitting energy is greater than the pairing energy. Pairing energy is energy required for the electrons to pair in an orbital before filling the higher energy state.
Splitting energy $\rangle $ Pairing energy
Hence the electrons pair up in the ${{t}_{2}}g$ orbital, So, the correct answer is “Option B”.
Note: If the given complex was tetrahedral i.e. with four ligands surrounding the central metal atom then the orbital with lower energy is – e and higher energy is ${{t}_{2}}$. So according to the question requirement the orbital splitting should be taken care of, and if the ligand was water, Cl etc. then they are weak ligands and splitting energy is less than the pairing energy. Hence the electrons will move to the higher orbital state without pairing.
${{t}_{2}}g$ should be filled with at least one electron in each orbitals to fill up in the higher ${{e}_{g}}$ orbital.
Complete step by step answer:
In the question it is given, a complex of Co. Co is the central metal atom and the cyanide ions are the ligands that are surrounding the Co atom, there are six cyanide ions and hence we can say that it is an octahedral complex.
- According to the spectrochemical series the cyanide ion is one of the strongest ligands carrying a charge of -1.
- And now we have to know that the Co is in which oxidation state, either we can find through the calculation method but here it is already given in the options that it is a $3{{d}^{6}}$ system.
- If it wasn’t given, then we calculate the oxidation State and find the ${{d}^{n}}$ system to which the complex belongs to.
For that in the complex give the oxidation number of Co as x.
${{\left[ Co{{\left( CN \right)}_{6}} \right]}^{3-}}$-oxidation state- $x+\left( -1 \right)(6)=-3$, x = 3
So here Co is in +3 oxidation state.
The electronic configuration of Co is -$\left[ Ar \right]3{{d}^{7}}4{{s}^{2}}$
In +3 state, it will have a configuration as $\left[ Ar \right]3{{d}^{6}}$
So the system is ${{d}^{6}}$ system.
- Now we know that in d orbitals are divided into 2 levels, ${{t}_{2}}g$ having a set of 3 orbitals ${{d}_{xy}},{{d}_{yz}},{{d}_{xz}}$ and ${{e}_{g}}$ with two orbitals ${{d}_{{{x}^{2}}-{{y}^{2}}}},{{d}_{{{z}^{2}}}}$
- As this complex is an octahedral complex, ${{t}_{2}}g$ will be the orbital with lower energy level and ${{e}_{g}}$ is the higher energy level
- As $C{{N}^{-}}$ is a strong ligand it splits up, ${{t}_{2}}g$ and ${{e}_{g}}$ in a greater energy, splitting energy is greater than the pairing energy. Pairing energy is energy required for the electrons to pair in an orbital before filling the higher energy state.
Splitting energy $\rangle $ Pairing energy
Hence the electrons pair up in the ${{t}_{2}}g$ orbital, So, the correct answer is “Option B”.
Note: If the given complex was tetrahedral i.e. with four ligands surrounding the central metal atom then the orbital with lower energy is – e and higher energy is ${{t}_{2}}$. So according to the question requirement the orbital splitting should be taken care of, and if the ligand was water, Cl etc. then they are weak ligands and splitting energy is less than the pairing energy. Hence the electrons will move to the higher orbital state without pairing.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




