
The correct order of boiling point is:
A.$HF\, > HI > HBr > HCl$
B.$HF > HBr > HI > HCl$
C.$HCl > HBr > HI > HF$
D.$HCl > HI > HBr > HF$
Answer
552.3k+ views
Hint: As we move down the group the attraction is greater or compound forms are having strong bonds thus they should have larger boiling points then the above compounds. But here there is a phenomenon happening between electronegative elements by which it has the highest boiling point. Find out the phenomenon and then write down the order.
Complete step-by-step answer:In the whole periodic table, we have fluorine as the most electronegative element with it there are two also. These electronegative elements form hydrogen bonding with hydrogen atoms which may be present in the same molecule and may be in other molecules. If hydrogen bonding occurs within a molecule it is called intramolecular hydrogen bonding but where the molecules are different then the bonding is called intermolecular bonding.
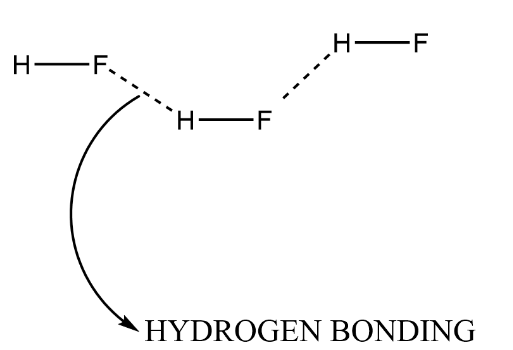
Whenever a compound hydrogen bonding is present, it makes the bond exceptionally strong thus we need a higher amount of energy to break that bond and hence according to the above question it will give a high boiling point because at boiling also certain bonds get broken. We know the halides are such that there will be an increase in size so the group $HI$ structure will have stronger bonds thus, it possesses a high boiling point and after that $HBr$ and then $HCl$ . There is an exceptional behaviour due to hydrogen bonding that $HF$ will make the strongest bond and possess the highest boiling point.
Option A will be correct.
Note:A question may be in your mind that why only fluorine is having hydrogen bonding and other halides don’t. This is because of the high electronegativity that fluorine possess and all other halides like iodine, chlorine and bromine don’t possess. The halides have stronger structural formation down the group; it means hydrogen iodide will have the highest among the last three halides.
Complete step-by-step answer:In the whole periodic table, we have fluorine as the most electronegative element with it there are two also. These electronegative elements form hydrogen bonding with hydrogen atoms which may be present in the same molecule and may be in other molecules. If hydrogen bonding occurs within a molecule it is called intramolecular hydrogen bonding but where the molecules are different then the bonding is called intermolecular bonding.

Whenever a compound hydrogen bonding is present, it makes the bond exceptionally strong thus we need a higher amount of energy to break that bond and hence according to the above question it will give a high boiling point because at boiling also certain bonds get broken. We know the halides are such that there will be an increase in size so the group $HI$ structure will have stronger bonds thus, it possesses a high boiling point and after that $HBr$ and then $HCl$ . There is an exceptional behaviour due to hydrogen bonding that $HF$ will make the strongest bond and possess the highest boiling point.
Option A will be correct.
Note:A question may be in your mind that why only fluorine is having hydrogen bonding and other halides don’t. This is because of the high electronegativity that fluorine possess and all other halides like iodine, chlorine and bromine don’t possess. The halides have stronger structural formation down the group; it means hydrogen iodide will have the highest among the last three halides.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




