
The correct order of electron density in the aromatic ring of the given compounds is:
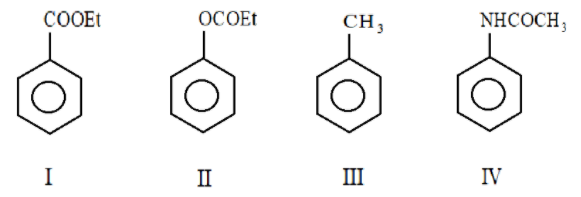
A. $H - N - $
B. $I > II > III > IV$
C. $IV > II > I > III$
D. $IV > II > III > I$

Answer
570k+ views
Hint: We know that electron density of a benzene ring will increase if electrons will be donated to it. The electron donating group such as the alkyl group donates electrons to the benzene ring and hence increases the density. On the other hand the electron withdrawing groups such as the nitro group extracts electrons from the benzene ring and in turn also decreases the density of the ring.
Complete answer:
In the first ring:
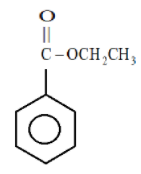
Here the group attached to the benzene ring is the electron withdrawing group because we know that oxygen is more electronegative than the carbon atom so it will extract electrons from the carbon atom, hence the as carbon is now in need of more electrons it will therefore withdraw electrons from the benzene ring and hence the electron density on benzene ring will decrease.
Now in the second ring
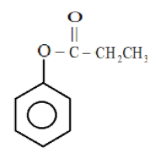
Here we see that the lone pair of electrons present on oxygen are delocalized and there will come in resonance with the benzene ring thereby increasing the electron density of the ring.
In the third ring:
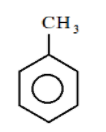
We see that here an alkyl group is directly attached to the benzene ring and we already know that the alkyl groups are the electron donating group, so the it will donate electrons to the benzene ring by $ + I$ effect and will increase the density of the benzene ring but as we know that the resonance effect is stronger than the $ + I$ effect therefore in this case the electron density of second ring will be more than the density of third ring.
In the fourth ring:
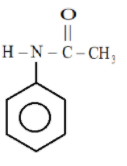
Here we see that the lone pair present on nitrogen are in resonance with the $C = O$ group and also with the benzene ring so the electron density will increase here too but as we know that oxygen is more electronegative than nitrogen, Nitrogen will therefore easily donate its electrons to the ring while oxygen will not give too easily so the electron density would be the highest in this case .
So, the correct order of electron density in aromatic ring is $IV > II > III > I$
Hence the correct answer is Option D.
Note:
$ + I$ is the one which is caused when a chemical compound which has a tendency to donate electrons, is introduced in the carbon chain. Electronegativity is the tendency of an atom to attract electrons towards itself in a chemical compound. The electronegativity increases on moving from left to right in a period and decreases on moving down the group.
Complete answer:
In the first ring:

Here the group attached to the benzene ring is the electron withdrawing group because we know that oxygen is more electronegative than the carbon atom so it will extract electrons from the carbon atom, hence the as carbon is now in need of more electrons it will therefore withdraw electrons from the benzene ring and hence the electron density on benzene ring will decrease.
Now in the second ring

Here we see that the lone pair of electrons present on oxygen are delocalized and there will come in resonance with the benzene ring thereby increasing the electron density of the ring.
In the third ring:

We see that here an alkyl group is directly attached to the benzene ring and we already know that the alkyl groups are the electron donating group, so the it will donate electrons to the benzene ring by $ + I$ effect and will increase the density of the benzene ring but as we know that the resonance effect is stronger than the $ + I$ effect therefore in this case the electron density of second ring will be more than the density of third ring.
In the fourth ring:

Here we see that the lone pair present on nitrogen are in resonance with the $C = O$ group and also with the benzene ring so the electron density will increase here too but as we know that oxygen is more electronegative than nitrogen, Nitrogen will therefore easily donate its electrons to the ring while oxygen will not give too easily so the electron density would be the highest in this case .
So, the correct order of electron density in aromatic ring is $IV > II > III > I$
Hence the correct answer is Option D.
Note:
$ + I$ is the one which is caused when a chemical compound which has a tendency to donate electrons, is introduced in the carbon chain. Electronegativity is the tendency of an atom to attract electrons towards itself in a chemical compound. The electronegativity increases on moving from left to right in a period and decreases on moving down the group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




