
The correct structure of ethylenediaminetetraacetic acid (EDTA) is:
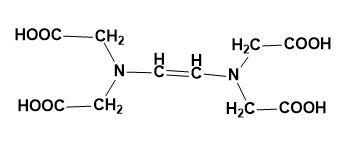
(A)
(B)
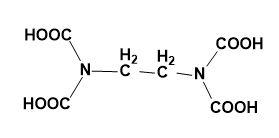
(C)
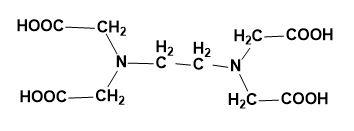
(D)
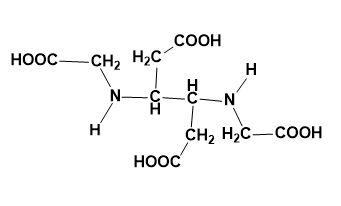




Answer
565.2k+ views
Hint:. EDTA (ethylenediaminetetraacetic acid) is a tetraprotic acid. As the name suggests, the structure will consist of ethane groups attached to the two amine groups which will result in the formation of ethylene diamine. Also, the four hydrogen atoms are replaced by the acetic acid groups resulting in complex structure.
- The molecular formula for EDTA is ${{C}_{10}}{{H}_{16}}{{N}_{2}}{{O}_{8}}$ .
Complete step by step answer:
Let us know about EDTA before determining its structure;
EDTA- Ethylenediaminetetraacetic acid is a complexing agent. It is a colourless and water-soluble compound in its salt form. It is a hexadentate ligand.
Structure- EDTA has four carboxylic acid groups and two amine groups with lone pairs of electrons on it. The ethane is attached to two amine groups making it to be called as ethylene diamine. Then the two hydrogens from the amine (each) are replaced by the acetic acid group which makes EDTA possible.
The structure of EDTA is:
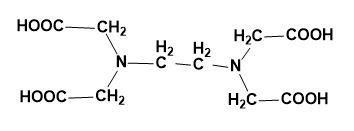
So, the correct answer is “Option C”.
Note: Do note that EDTA is an acid so it favours reactions in basic medium. It is actually a polyprotic acid as ethylenediaminetetraacetic acid (abbreviated as EDTA), can add two more hydrogens on the two nitrogen of the amine group.
- The molecular formula for EDTA is ${{C}_{10}}{{H}_{16}}{{N}_{2}}{{O}_{8}}$ .
Complete step by step answer:
Let us know about EDTA before determining its structure;
EDTA- Ethylenediaminetetraacetic acid is a complexing agent. It is a colourless and water-soluble compound in its salt form. It is a hexadentate ligand.
Structure- EDTA has four carboxylic acid groups and two amine groups with lone pairs of electrons on it. The ethane is attached to two amine groups making it to be called as ethylene diamine. Then the two hydrogens from the amine (each) are replaced by the acetic acid group which makes EDTA possible.
The structure of EDTA is:

So, the correct answer is “Option C”.
Note: Do note that EDTA is an acid so it favours reactions in basic medium. It is actually a polyprotic acid as ethylenediaminetetraacetic acid (abbreviated as EDTA), can add two more hydrogens on the two nitrogen of the amine group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




