
The covalency of sulphur in the ($S_8$) molecule is:
Answer
584.7k+ views
Hint: Atoms and molecules combine with each other to form the different compounds. The measure of the combining capacity of atoms or molecules is known as valency. Thus it gives the measure of the number of one atom combining with the other atom to form the bonds.
Complete step by step answer:
Sulphur is the element number 16 in the periodic table and belongs to group 16 and period 3 of the periodic table. The symbol of this element is S. It is a non- metal and is odourless and tasteless in nature. The native existence of sulphur is in the yellow colour crystalline solid. The crystal structure of sulphur is complex.
Covalency of an atom is the number of pairs of electrons it can share during the formation of the compound with the other atom, these electrons usually belong to the outermost shell of the atom. When an atom shares 1 electron, its covalency is equal to 1 and if it shares 2 electrons, its covalency is 2.
The structure of $S_8$ molecules is complex in nature. Sulphur shows the property of allotropy also. The existence of an element in two or more different physical forms is known as allotropy. The two more common allotropes of sulphur are rhombic ($alpha$-sulphur) and monoclinic ($beta$-sulphur) sulphur.
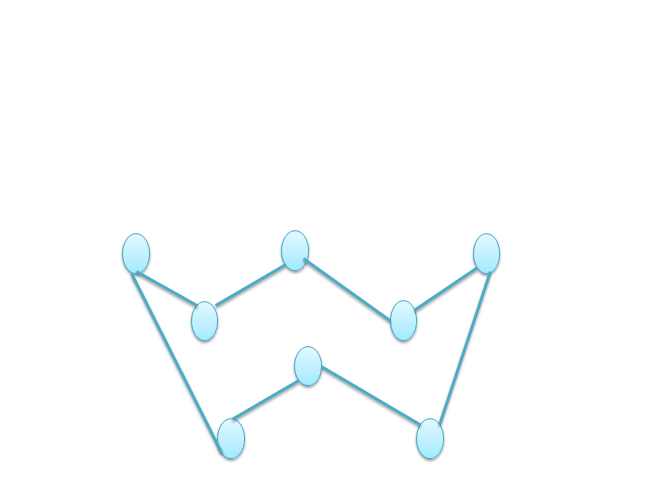
In the above diagram each circle depicts one sulphur atom.
The covalency of sulphur in $S_8$ molecule is 2 because of the S-S-S-S chain in the structure of the $S_8$ molecule due to which it can share only 2 electrons with the other molecules to form the bonds through sharing , the covalent bonds will be formed.
Note:
Sulphur compounds are toxic in nature and they have adverse effects on humans such as disturbance in the blood circulation, heart damage, reproductive failure etc. Although it is present in abundance in nature. Inhalation of the sulphur compounds results in suffocation.
Complete step by step answer:
Sulphur is the element number 16 in the periodic table and belongs to group 16 and period 3 of the periodic table. The symbol of this element is S. It is a non- metal and is odourless and tasteless in nature. The native existence of sulphur is in the yellow colour crystalline solid. The crystal structure of sulphur is complex.
Covalency of an atom is the number of pairs of electrons it can share during the formation of the compound with the other atom, these electrons usually belong to the outermost shell of the atom. When an atom shares 1 electron, its covalency is equal to 1 and if it shares 2 electrons, its covalency is 2.
The structure of $S_8$ molecules is complex in nature. Sulphur shows the property of allotropy also. The existence of an element in two or more different physical forms is known as allotropy. The two more common allotropes of sulphur are rhombic ($alpha$-sulphur) and monoclinic ($beta$-sulphur) sulphur.

In the above diagram each circle depicts one sulphur atom.
The covalency of sulphur in $S_8$ molecule is 2 because of the S-S-S-S chain in the structure of the $S_8$ molecule due to which it can share only 2 electrons with the other molecules to form the bonds through sharing , the covalent bonds will be formed.
Note:
Sulphur compounds are toxic in nature and they have adverse effects on humans such as disturbance in the blood circulation, heart damage, reproductive failure etc. Although it is present in abundance in nature. Inhalation of the sulphur compounds results in suffocation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




