
The critical temperature of a gas is the temperature.
A. above which it can no longer exist in gaseous state
B. above which cannot be liquified by pressure.
C. at which it solidifies
D. at which the volume of the gas becomes zero
Answer
586.8k+ views
Hint: In thermodynamics, the critical temperature of a substance can be defined as the highest – temperature at which the substance can exist as a liquid. The substance can no longer be liquefied, regardless of the amount of pressure applied to it. It is the highest temperature at which gaseous state starts condensation into liquid state. It is represented as ${T_0}$ e.g. the critical temperature of carbon dioxide gas is $30.38 C$ and it was discovered by the French physicist Charles Cagniard de la tour in $1852$.
Complete step by step answer:
The critical temperature can best be understood by observing a simple experiment. If a closed vessel is filled with a pure substance, partly liquid and partly vapour, so that the average density, the critical conditions can be achieved. As the temperature is raised, the vapour pressure increases, and the gas phase becomes denser, the liquid expands and becomes less dense until at the critical point, densities of the liquid and vapour become equal, eliminating the boundary between the two phases. If the average density at the start is too low, all the liquid will evaporate before the critical temperature is reached. If the initial average density is too high, the liquid will expand to fill the container.
${T_C} = \dfrac{{8a}}{{27Rb}}$
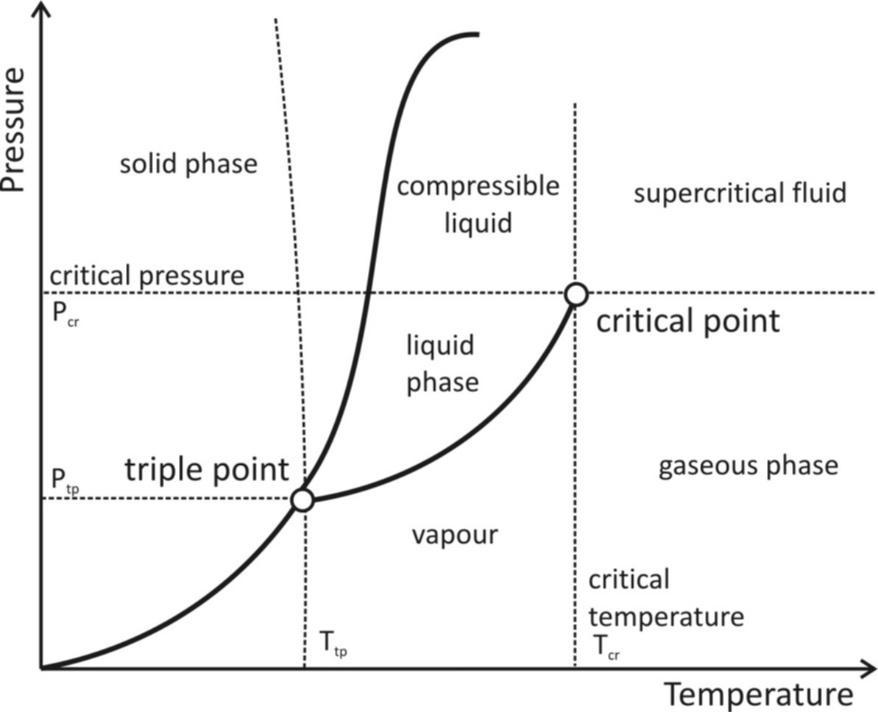
Where A and b are specific constants for each g are a graph describing the triple point and the critical point of a substance is provided below it can be noted that graph is plotted with pressure on the Y – axis and temperature on the X – axis. Therefore, the critical temperature can be obtained from the X – axis value of the critical point. The corresponding Y – axis value of the critical point, which is the pressure required to liquefy a substance at its critical temperature is known as the critical pressure of the substance.
The correct option is B – above which it cannot be liquefied by pressure
Note:
The critical temperature of a gas provides insight into the strength of the intermolecular forces of attraction that its particles are subject to e.g. A gaseous substance with relatively weak intermolecular forces will be harder to liquefy than a gaseous substance featuring stronger intermolecular forces of attraction. Therefore, the weaker the intermolecular forces, the lower the critical temperature.
Complete step by step answer:
The critical temperature can best be understood by observing a simple experiment. If a closed vessel is filled with a pure substance, partly liquid and partly vapour, so that the average density, the critical conditions can be achieved. As the temperature is raised, the vapour pressure increases, and the gas phase becomes denser, the liquid expands and becomes less dense until at the critical point, densities of the liquid and vapour become equal, eliminating the boundary between the two phases. If the average density at the start is too low, all the liquid will evaporate before the critical temperature is reached. If the initial average density is too high, the liquid will expand to fill the container.
${T_C} = \dfrac{{8a}}{{27Rb}}$
Where A and b are specific constants for each g are a graph describing the triple point and the critical point of a substance is provided below it can be noted that graph is plotted with pressure on the Y – axis and temperature on the X – axis. Therefore, the critical temperature can be obtained from the X – axis value of the critical point. The corresponding Y – axis value of the critical point, which is the pressure required to liquefy a substance at its critical temperature is known as the critical pressure of the substance.

The correct option is B – above which it cannot be liquefied by pressure
Note:
The critical temperature of a gas provides insight into the strength of the intermolecular forces of attraction that its particles are subject to e.g. A gaseous substance with relatively weak intermolecular forces will be harder to liquefy than a gaseous substance featuring stronger intermolecular forces of attraction. Therefore, the weaker the intermolecular forces, the lower the critical temperature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




