
The crystal lattice of $NaCl$ is:
A. Face-centered Cubic lattice
B. Body-centered cubic lattice
C. Simple cubic lattice
D. Hexagonal close packing
Answer
587.1k+ views
Hint: A unit cell which has a lattice point at every face centre in addition to the lattice point at every corner of the cubic unit cell is known as face centered cubic unit cell. In this case there are eight atoms at the eight corners of the unit cell and six atoms at the centre of six faces of the unit cell.
Complete answer:
In the case of a $NaCl$ structure, the geometry is cubic close packing or face centered cubic geometry. In this geometry, the sodium ions are present at every octahedral void and the chloride ions are present at every element of a cubic close packed structure. The coordination number of the sodium atoms and the chlorine atoms are in the ratio of 6:6. The number of formulas per unit cell is equal to:
$4N{a^ + } + 4C{l^ - } \to 4NaCl(4)$
The structure of sodium chloride crystal lattice is as follows:
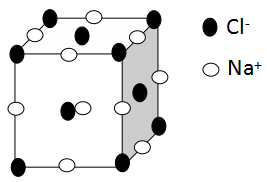
As we can see from the diagram, the sodium ions occupy the edge of the unit cell and the chloride ions occupy the corners and the centre of the unit cell.
The packing efficiency of a $NaCl$ structure is $74\% $ and the remaining $26\% $ is void space inside the cubic cell. The relation between the edge length ‘a’ and the radius of the atoms ‘r’ is:
$r = \dfrac{a}{{2\sqrt 2 }}$ . The number of atoms per unit cell is 4 as mentioned above.
So, the correct answer is Option A .
Note:
Rock salt ($NaCl$) is an ionic compound that occurs naturally in the form of white crystals. It is extracted either from the mineral form or the evaporation of seawater. The structure of $NaCl$ is formed by repeating the face centered cubic unit cell. It has \[1:1\] stoichiometry ratio of \[Na:Cl\] with a molar mass of \[58.4{\text{ }}g/mol\].
Complete answer:
In the case of a $NaCl$ structure, the geometry is cubic close packing or face centered cubic geometry. In this geometry, the sodium ions are present at every octahedral void and the chloride ions are present at every element of a cubic close packed structure. The coordination number of the sodium atoms and the chlorine atoms are in the ratio of 6:6. The number of formulas per unit cell is equal to:
$4N{a^ + } + 4C{l^ - } \to 4NaCl(4)$
The structure of sodium chloride crystal lattice is as follows:

As we can see from the diagram, the sodium ions occupy the edge of the unit cell and the chloride ions occupy the corners and the centre of the unit cell.
The packing efficiency of a $NaCl$ structure is $74\% $ and the remaining $26\% $ is void space inside the cubic cell. The relation between the edge length ‘a’ and the radius of the atoms ‘r’ is:
$r = \dfrac{a}{{2\sqrt 2 }}$ . The number of atoms per unit cell is 4 as mentioned above.
So, the correct answer is Option A .
Note:
Rock salt ($NaCl$) is an ionic compound that occurs naturally in the form of white crystals. It is extracted either from the mineral form or the evaporation of seawater. The structure of $NaCl$ is formed by repeating the face centered cubic unit cell. It has \[1:1\] stoichiometry ratio of \[Na:Cl\] with a molar mass of \[58.4{\text{ }}g/mol\].
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




