
The dipole moment of $C{O_2}$ is zero, which implies that:-
(A) Carbon and oxygen have equal electronegativity.
(B) Carbon has no polar bond.
(C) $C{O_2}$ is a linear molecule.
(D) Carbon has bond moments of zero value.
Answer
585.6k+ views
Hint: The dipole moment is based on the direction of movement of electrons. Electrons In case of covalent bond will move when there is electronegativity difference.
Complete step by step answer:
Dipole moment arises when there is separation of charge in the system. This is mainly observed in ionic compounds. In cases of covalent compounds, dipole moment is observed when there is difference in the electronegativity between two chemically bonded atoms.
A bond dipole moment measures the polarity of the molecule. It is a vector quantity which means it depends on both direction and magnitude.
Dipole moment is the product of magnitude of charge and distance between the centers of the positive and negative charge present on two different atoms. Mathematically we can represent it as follows:
$\mu = \delta .d$
$\mu $= dipole moment.
$\delta $= magnitude of the partial charges
d = distance between${\delta ^ + }$ and ${\delta ^ - }$
The $\delta $charges represent two electric charges which are equal in magnitude but have opposite signs. These are separated by the distance (d).
As the bond dipole moment is a vector quantity, the direction of the dipole moment is parallel to the bond axis. Here the arrows are drawn to represent dipole moments. The head of the arrow is pointed towards the negative charge.
As per the given information, the dipole moment of $C{O_2}$ is zero. As there is electronegativity difference the bonded electrons move towards the more electronegative atom. Here, oxygen is more electronegative than carbon. In this case, the electrons will move towards a more electronegative oxygen atom. Therefore, oxygen will carry while carbon will ${\delta ^ - }$ carry charge. Thus the direction of individual dipole moments will be from carbon towards oxygen atoms. The dipole moment will be zero only in one case which is when the dipole moment will cancel out each other. Thus the dipole moment can cancel out each other when both the oxygen atoms are at the opposite side of the carbon atom. Thus $C{O_2}$ is a linear molecule.
The bond structure of $C{O_2}$ is as follows:
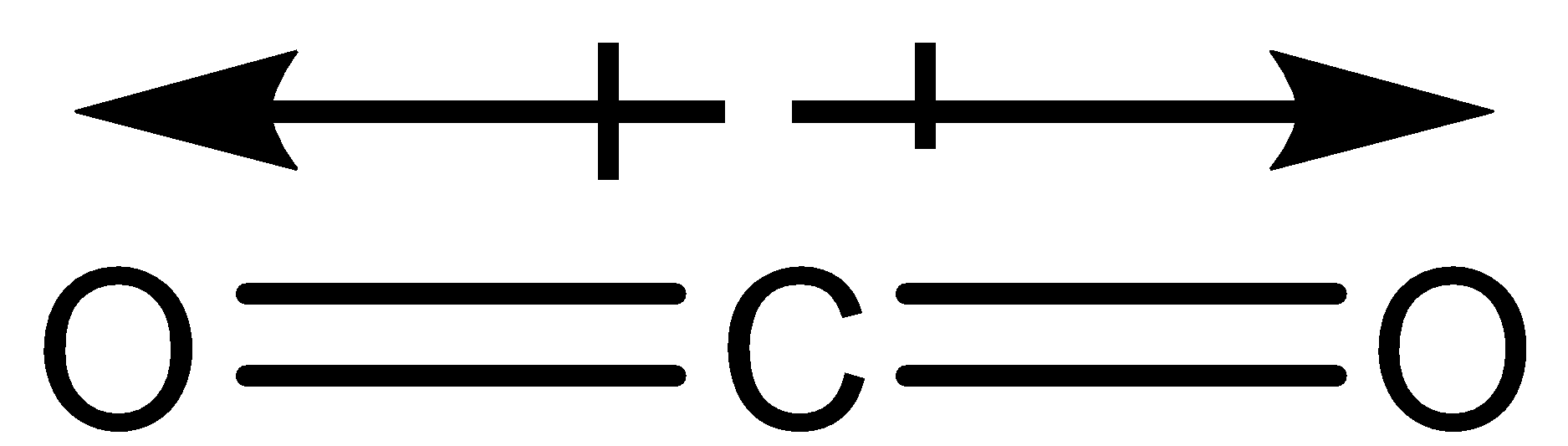
So, the correct answer is “Option C”.
Note: In chemistry the dipole moment is represented by a slight difference of the arrow symbol. The arrow over here consists of a cross at the tail. The cross will be on the positive center and the arrowhead will be pointed at the negative center.
Complete step by step answer:
Dipole moment arises when there is separation of charge in the system. This is mainly observed in ionic compounds. In cases of covalent compounds, dipole moment is observed when there is difference in the electronegativity between two chemically bonded atoms.
A bond dipole moment measures the polarity of the molecule. It is a vector quantity which means it depends on both direction and magnitude.
Dipole moment is the product of magnitude of charge and distance between the centers of the positive and negative charge present on two different atoms. Mathematically we can represent it as follows:
$\mu = \delta .d$
$\mu $= dipole moment.
$\delta $= magnitude of the partial charges
d = distance between${\delta ^ + }$ and ${\delta ^ - }$
The $\delta $charges represent two electric charges which are equal in magnitude but have opposite signs. These are separated by the distance (d).
As the bond dipole moment is a vector quantity, the direction of the dipole moment is parallel to the bond axis. Here the arrows are drawn to represent dipole moments. The head of the arrow is pointed towards the negative charge.
As per the given information, the dipole moment of $C{O_2}$ is zero. As there is electronegativity difference the bonded electrons move towards the more electronegative atom. Here, oxygen is more electronegative than carbon. In this case, the electrons will move towards a more electronegative oxygen atom. Therefore, oxygen will carry while carbon will ${\delta ^ - }$ carry charge. Thus the direction of individual dipole moments will be from carbon towards oxygen atoms. The dipole moment will be zero only in one case which is when the dipole moment will cancel out each other. Thus the dipole moment can cancel out each other when both the oxygen atoms are at the opposite side of the carbon atom. Thus $C{O_2}$ is a linear molecule.
The bond structure of $C{O_2}$ is as follows:
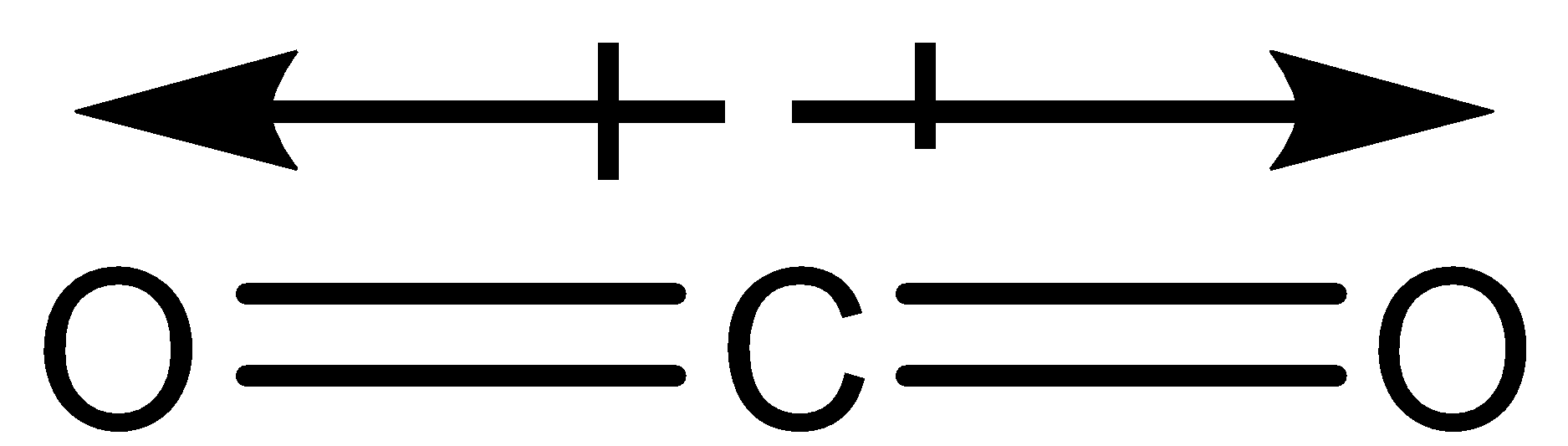
So, the correct answer is “Option C”.
Note: In chemistry the dipole moment is represented by a slight difference of the arrow symbol. The arrow over here consists of a cross at the tail. The cross will be on the positive center and the arrowhead will be pointed at the negative center.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




