
The ease of dehydration of the following alcohols will be in the order:
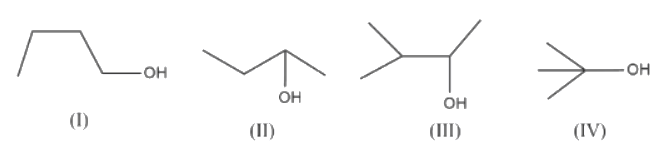
a.) IV > III > II > I
b.) I > II > III > IV
c.) IV > II > III > I
d.) II > IV > III > I

Answer
600k+ views
Hint: The stabler the alkene formed as a result of the dehydration of the alcohol, the greater the ease of its dehydration of said alcohol. With this logic in mind, try to solve the given question so as to ensure that the given rule is followed.
Complete answer:
Let us first establish what the dehydration of alcohol really is and what it results in.
The dehydration of alcohols results in the production of alkenes which can be affected by two common methods:
By passing the vapours of an alcohol over heated alumina.
By heating an alcohol with concentrated mineral acid, such as concentrated \[{{H}_{2}}S{{O}_{4}}\] or concentrated \[{{H}_{3}}P{{O}_{4}}\]. Anhydrous zinc chloride can also be used as a dehydrating agent.
By passing the vapours of an alcohol over alumina (\[A{{l}_{2}}{{O}_{3}}\]) at 623 K (350°C).
The dehydration of alcohols is a process in which alcohols undergo E1 or E2 mechanisms to lose water and form a double bond. The dehydration reaction of alcohols to generate alkene proceeds by heating the alcohols in the presence of a strong acid, such as sulfuric or phosphoric acid, at high temperatures.
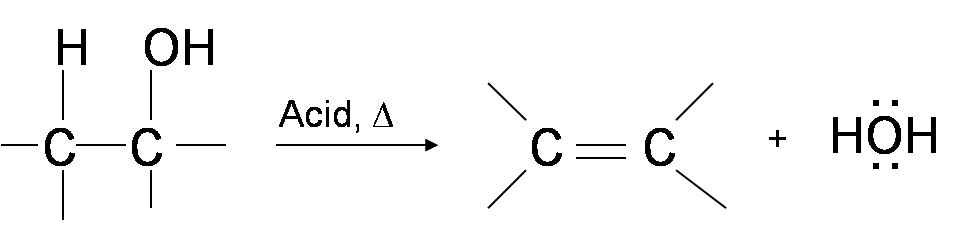 The order of the ease of dehydration of alcohols is: tertiary > secondary > primary. Secondary and tertiary alcohols are best dehydrated by dilute sulfuric acid.
The order of the ease of dehydration of alcohols is: tertiary > secondary > primary. Secondary and tertiary alcohols are best dehydrated by dilute sulfuric acid.
With this in mind, let us now look at the given alcohol. From the given alcohol, IV is a tertiary alcohol and III is a secondary alcohol. Therefore, these alcohols exhibit the most ease of dehydration of the given substances, with IV more so than III.
Now, I and II are both primary alcohols. Therefore, let us now compare the stability of the alkenes formed as a result of dehydration. We find that II results in 2-butene whereas I results in 1-butene. Now, we know that an alkyl group has a positive inductive effect. They are slightly electron releasing. They push electrons towards the double bond which helps stabilize it. So, the more substituted the C's of the double bond the more stable the bond. That is why 2- butene is more stable than 1- butene.
Thus, we can safely conclude that the correct order of ease of dehydration is IV > III > II > I. Therefore, the answer is a).
Note: If the reaction is not sufficiently heated, the alcohols do not dehydrate to form alkenes, but react with one another to form ethers (e.g., the Williamson Ether Synthesis). Be very wary of this phenomenon when trying to write this reaction.
Complete answer:
Let us first establish what the dehydration of alcohol really is and what it results in.
The dehydration of alcohols results in the production of alkenes which can be affected by two common methods:
By passing the vapours of an alcohol over heated alumina.
By heating an alcohol with concentrated mineral acid, such as concentrated \[{{H}_{2}}S{{O}_{4}}\] or concentrated \[{{H}_{3}}P{{O}_{4}}\]. Anhydrous zinc chloride can also be used as a dehydrating agent.
By passing the vapours of an alcohol over alumina (\[A{{l}_{2}}{{O}_{3}}\]) at 623 K (350°C).
The dehydration of alcohols is a process in which alcohols undergo E1 or E2 mechanisms to lose water and form a double bond. The dehydration reaction of alcohols to generate alkene proceeds by heating the alcohols in the presence of a strong acid, such as sulfuric or phosphoric acid, at high temperatures.
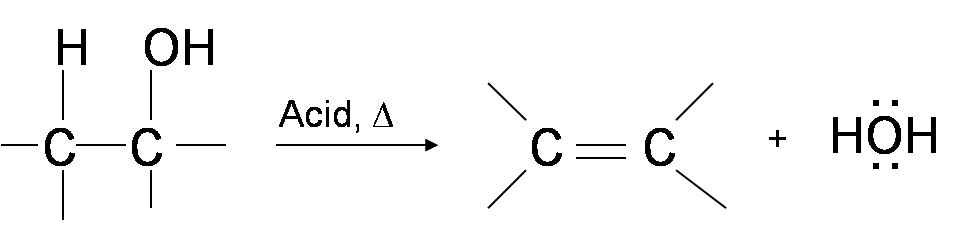
With this in mind, let us now look at the given alcohol. From the given alcohol, IV is a tertiary alcohol and III is a secondary alcohol. Therefore, these alcohols exhibit the most ease of dehydration of the given substances, with IV more so than III.
Now, I and II are both primary alcohols. Therefore, let us now compare the stability of the alkenes formed as a result of dehydration. We find that II results in 2-butene whereas I results in 1-butene. Now, we know that an alkyl group has a positive inductive effect. They are slightly electron releasing. They push electrons towards the double bond which helps stabilize it. So, the more substituted the C's of the double bond the more stable the bond. That is why 2- butene is more stable than 1- butene.
Thus, we can safely conclude that the correct order of ease of dehydration is IV > III > II > I. Therefore, the answer is a).
Note: If the reaction is not sufficiently heated, the alcohols do not dehydrate to form alkenes, but react with one another to form ethers (e.g., the Williamson Ether Synthesis). Be very wary of this phenomenon when trying to write this reaction.
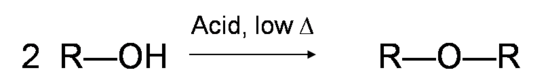
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




