
The edge length of the rock salt type unit cell is 508 pm. If the radius of the cation is 110 pm, the radius of the anion assuming NaCl type structure is:
A. 144 pm
B. 398 pm
C. 288 pm
D. 618 pm
Answer
582.3k+ views
Hint: The classification of various structures can be done by finding the radius ratio of the cation and anion. Depending upon the range of the radius ratio, we can classify the structures. The radius ratio (also known as radius ratio rule), is the ratio of the ionic radius of the cation to the ionic radius of the anion in a cation-anion compound.
Complete step by step answer:
The radius ratio of cation and anion is given by \[\dfrac{{r(cation)}}{{r(anion)}}\] .
For NaCl structure, the radius ratio lies between 0.414 to 0.732.
Thus, we have a NaCl structure.
NaCl has a cubic unit cell. It is also known as rock salt and it is an essential compound our body uses to absorb and transport nutrients, to maintain blood pressure, and to maintain the right balance of fluid. It is a crystal structure with a face-centered cubic lattice and two atoms in the basis. The number of atoms in the NaCl structure is as shown:
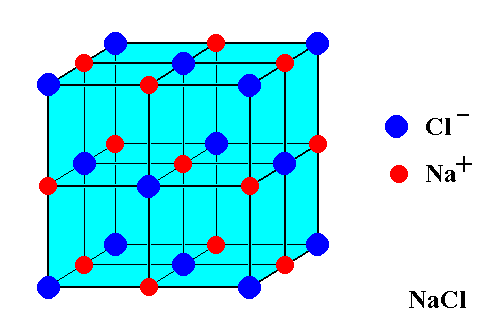
This structure of NaCl is also known as rock salt structure. In the case of rock salt structure, the edge length of the lattice is equal to the sum of cation and anion radii. The formula is
\[2 \times \left[ {r(cation) + r(anion)} \right] = a\] , where, a is the edge length of the lattice.
Now according to the question, but the given values and find out the value of the radius of anion as follows,
\[
2 \times \left[ {r(cation) + r(anion)} \right] = a \\
2 \times \left[ {110 + r(anion)} \right] = 508 \\
\left[ {110 + r(anion)} \right] = \dfrac{{508}}{2} \\
\left[ {110 + r(anion)} \right] = 254 \\
\left[ {r(anion)} \right] = 144 \\
\]
So, the radius of the anion assuming NaCl type structure is 144 pm.
Therefore, the correct answer is, A
Note:The ions in a compound such as sodium chloride are arranged in a lattice structure. The ions cannot move to conduct the electric current. But when an ionic compound melts, the charged ions are free to move. Therefore, molten ion compounds conduct electricity.
Complete step by step answer:
The radius ratio of cation and anion is given by \[\dfrac{{r(cation)}}{{r(anion)}}\] .
For NaCl structure, the radius ratio lies between 0.414 to 0.732.
Thus, we have a NaCl structure.
NaCl has a cubic unit cell. It is also known as rock salt and it is an essential compound our body uses to absorb and transport nutrients, to maintain blood pressure, and to maintain the right balance of fluid. It is a crystal structure with a face-centered cubic lattice and two atoms in the basis. The number of atoms in the NaCl structure is as shown:

This structure of NaCl is also known as rock salt structure. In the case of rock salt structure, the edge length of the lattice is equal to the sum of cation and anion radii. The formula is
\[2 \times \left[ {r(cation) + r(anion)} \right] = a\] , where, a is the edge length of the lattice.
Now according to the question, but the given values and find out the value of the radius of anion as follows,
\[
2 \times \left[ {r(cation) + r(anion)} \right] = a \\
2 \times \left[ {110 + r(anion)} \right] = 508 \\
\left[ {110 + r(anion)} \right] = \dfrac{{508}}{2} \\
\left[ {110 + r(anion)} \right] = 254 \\
\left[ {r(anion)} \right] = 144 \\
\]
So, the radius of the anion assuming NaCl type structure is 144 pm.
Therefore, the correct answer is, A
Note:The ions in a compound such as sodium chloride are arranged in a lattice structure. The ions cannot move to conduct the electric current. But when an ionic compound melts, the charged ions are free to move. Therefore, molten ion compounds conduct electricity.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




