
The electrolytic decomposition of water gives ${H_2}$ and ${O_2}$ in the ratio of:
A. $1:2$ by volume
B. $2:1$ by volume
C. $8:1$ by mass
D. $1:2$ by mass
Answer
507.6k+ views
Hint: An electrolytic decomposition reaction is a type of reaction in which the activation energy for the decomposition of the reactant is provided in the form of electrical energy. The most common example of electrolytic decomposition is electrolysis of water in which it splits into its constituent elements.
Complete answer:
According to Avogadro’s hypothesis, the equal volumes of any gas at the same temperature and pressure consist of the same number of particles. Since the total volume that a gas occupies usually consists of empty space among the particles of gas, so the actual size of the particles is negligible. Thus, the number of molecules in a specific volume of an ideal gas is considered as independent of their size or the molar mass of the gas.
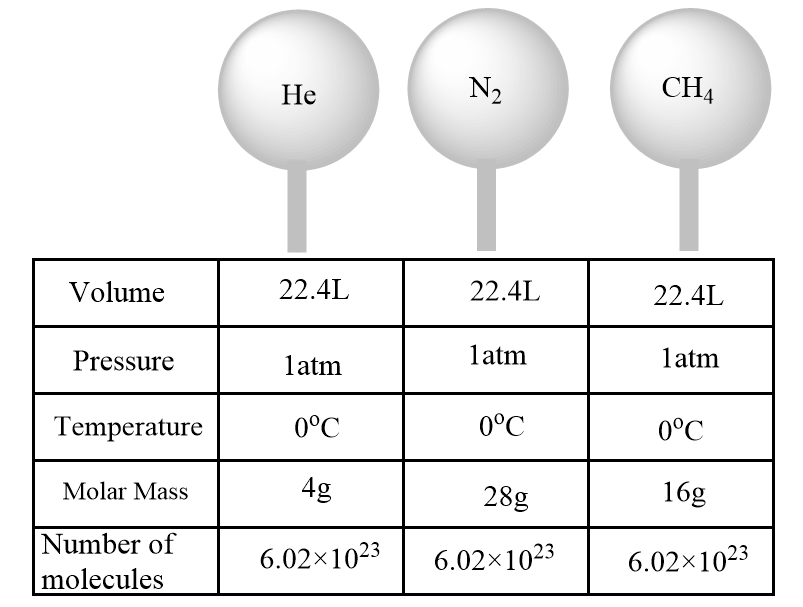
The given figure shows that at standard temperature and pressure, the equal number of molecules of gases are present irrespective of their molar mass.
Now, as per question the electrolytic decomposition of water takes place as per following reaction:
$2{H_2}O \to 2{H_2} + {O_2}$
From the reaction it is clearly observed that on electrolytic decomposition of water, two volumes of hydrogen gas and one volume of oxygen gas is formed respectively. Therefore, the ${H_2}$ and ${O_2}$ gases are formed in the ratio of $2:1$ by volume during the process.
So, the correct answer is “Option B”.
Note:
Always remember that the calculation of volume-volume ratios in a chemical reaction are based on Avogadro’s hypothesis according to which the volume of the gas is directly proportional to the number of particles it contains. Also, the pressures and temperatures of the gases (STP conditions for the given case) involved in the volume-volume ratio must be the same.
Complete answer:
According to Avogadro’s hypothesis, the equal volumes of any gas at the same temperature and pressure consist of the same number of particles. Since the total volume that a gas occupies usually consists of empty space among the particles of gas, so the actual size of the particles is negligible. Thus, the number of molecules in a specific volume of an ideal gas is considered as independent of their size or the molar mass of the gas.

The given figure shows that at standard temperature and pressure, the equal number of molecules of gases are present irrespective of their molar mass.
Now, as per question the electrolytic decomposition of water takes place as per following reaction:
$2{H_2}O \to 2{H_2} + {O_2}$
From the reaction it is clearly observed that on electrolytic decomposition of water, two volumes of hydrogen gas and one volume of oxygen gas is formed respectively. Therefore, the ${H_2}$ and ${O_2}$ gases are formed in the ratio of $2:1$ by volume during the process.
So, the correct answer is “Option B”.
Note:
Always remember that the calculation of volume-volume ratios in a chemical reaction are based on Avogadro’s hypothesis according to which the volume of the gas is directly proportional to the number of particles it contains. Also, the pressures and temperatures of the gases (STP conditions for the given case) involved in the volume-volume ratio must be the same.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




