
The electromeric effect in organic compounds is a:
A. Temporary effect
B. Permanent effect
C. Temporary or permanent effect
D. All of the above
Answer
584.1k+ views
Hint: The complete transfer of shared pi electrons in multiple bonds to other compounds in the presence of a reagent is called electromeric effect. Nothing but the movement of electrons from one compound having pi electrons to another atom in a different compound in the presence of a reagent.
Complete step by step answer:
- Electromeric effect can be represented with a symbol E.
- We can observe electromeric effects in organic compounds containing multiple bonds.
- There are two types of electromeric effects. +E and –E effect.
- In +E effect the pi electrons transfer towards attacking reagent and in –E effect the pi electrons transfer away from attacking reagent.
- Means in the presence of attacking reagent only +E or –E effects take place.
- Therefore the electromeric effect is a temporary effect.
So, the correct option is A.
Additional information:
- An example for +E effect is as follows.

- We can see clearly that in the above reaction pi electrons are transferred from one molecule to another because of the presence of hydrogen ions.
- An example for –E effect is as follows.
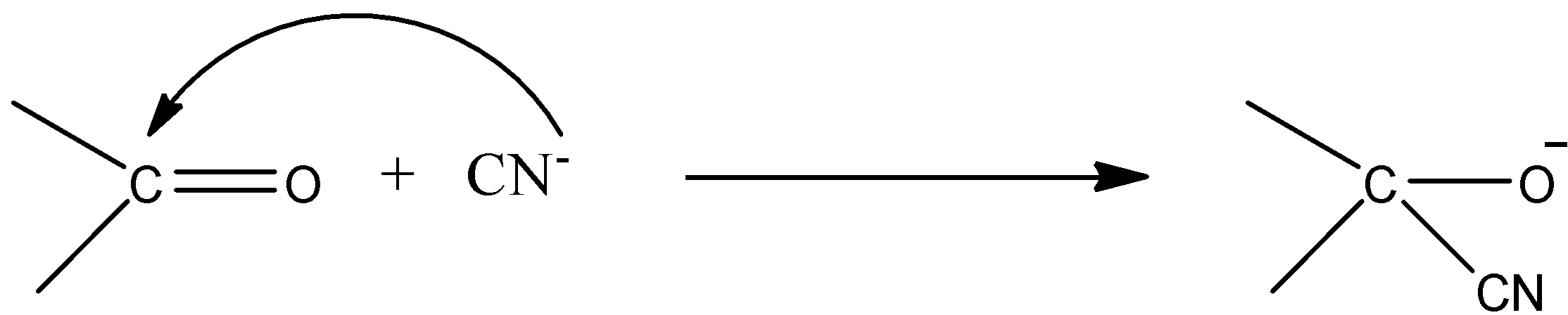
- We can see clearly that the transfer of electrons towards the multiple bond in the presence of an external reagent.
Note: For suppose if there is no external reagent then there is no electromeric effect. Means external reagents only play a big role in electromeric effect whether it is +E effect or –Affect. Electromeric effect will occur in the molecules containing double or triple bonds.
Complete step by step answer:
- Electromeric effect can be represented with a symbol E.
- We can observe electromeric effects in organic compounds containing multiple bonds.
- There are two types of electromeric effects. +E and –E effect.
- In +E effect the pi electrons transfer towards attacking reagent and in –E effect the pi electrons transfer away from attacking reagent.
- Means in the presence of attacking reagent only +E or –E effects take place.
- Therefore the electromeric effect is a temporary effect.
So, the correct option is A.
Additional information:
- An example for +E effect is as follows.

- We can see clearly that in the above reaction pi electrons are transferred from one molecule to another because of the presence of hydrogen ions.
- An example for –E effect is as follows.
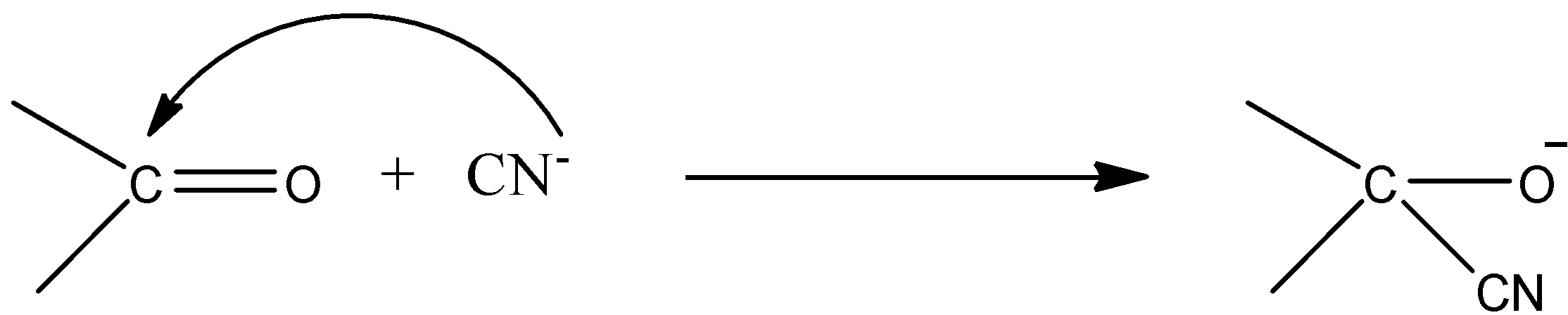
- We can see clearly that the transfer of electrons towards the multiple bond in the presence of an external reagent.
Note: For suppose if there is no external reagent then there is no electromeric effect. Means external reagents only play a big role in electromeric effect whether it is +E effect or –Affect. Electromeric effect will occur in the molecules containing double or triple bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




